D35.1 Functional Groups and Acidity/Basicity
Functional groups are specific structure moieties that have similar chemical properties whenever it is present in a molecule. Hence, we can consider certain functional groups as being acidic while others as basic. Hence, identifying the functional group(s) present in a molecule helps in quickly estimating that molecule’s reactivity.
Activity: Carboxylic Acid
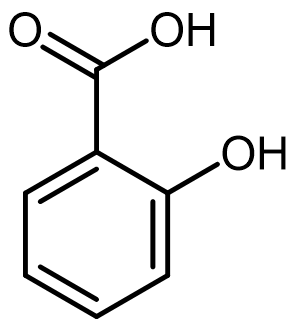 Many pharmaceutical molecules contain the carboxylic acid (-COOH) functional group, which exhibits acidic behavior in aqueous environments.
Many pharmaceutical molecules contain the carboxylic acid (-COOH) functional group, which exhibits acidic behavior in aqueous environments.
For an example, consider the salicylic acid molecule shown at right. Draw the overall reaction between salicyclic acid and water, show clearly the product molecules formed.
Draw in your notebook, then left-click here.
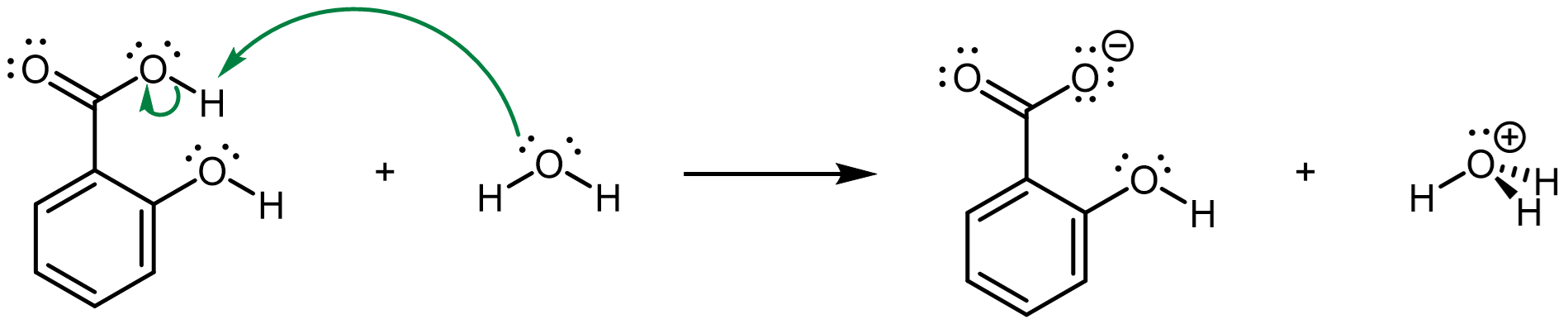
The conjugate base of salicyclic acid is salicylate, containing the carboxylate (-COO¯) functional group, which is the conjugate base of the carboxylic acid functional group.
Activity: Amine
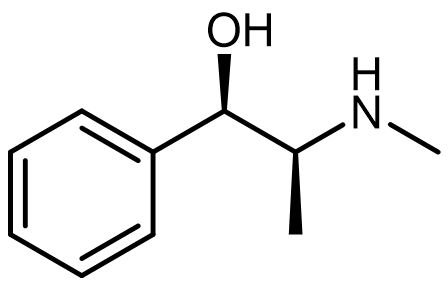 Many pharmaceutical molecules contain the amine functional group, which exhibits basic behavior in aqueous environments.
Many pharmaceutical molecules contain the amine functional group, which exhibits basic behavior in aqueous environments.
For an example, consider the ephedrine molecule shown at right. Draw the overall reaction between ephedrine and water, show clearly the product molecules formed.
Draw in your notebook, then left-click here.
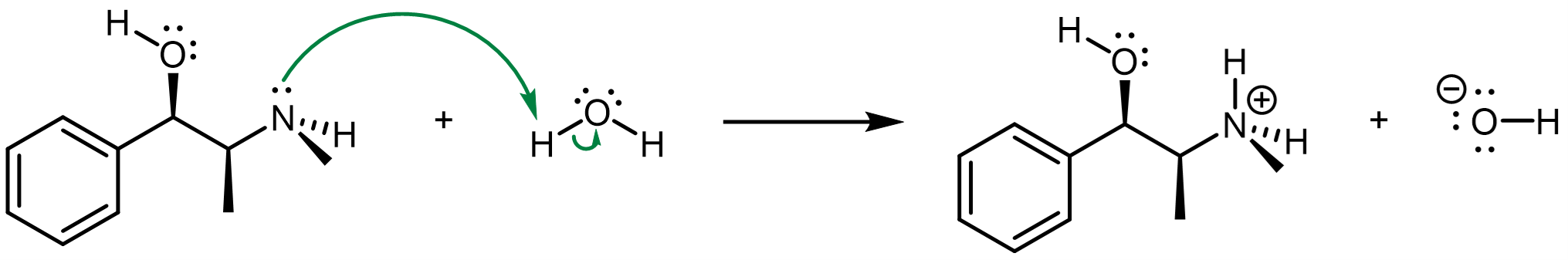
What makes one functional group acidic and another basic? Why is it that, in both examples above, the alcohol functional group appear to be neither acidic nor basic? Acid-base reactions, like many other chemical reactions, involve breaking and forming bonds. Hence, we can use our chemical understanding of molecular structure to understand what makes some acids (or bases) stronger than others.

