D7.1 Hydrocarbons: Why Use Lewis Structures?
Let’s start with what molecules actually look like—well, our best guess. Shown below is space-filling models of simple molecules made only of carbon and hydrogen: methane (CH4), ethane (C2H6), and propane (C3H8). These are all examples of hydrocarbons—molecules composed entirely of carbon and hydrogen atoms.
As you examine their structures, some patterns should stand out:
- Carbon (dark atoms) tends to form four connections.
- Hydrogen (light atoms) always forms one connection.
- The shapes are not flat—they’re three-dimensional, with atoms arranged to minimize repulsion between electron regions.
These 3D space-filling representations give you a tangible sense of how atoms pack together. They show approximate relative sizes, angles, and how tightly atoms are bonded. But they’re also hard to sketch and annotate, especially as molecules get larger or more complex.
To make structure easier to communicate, chemists use a 2D system called Lewis structures—a symbolic shorthand for representing molecules in two dimensions. Lewis structures represent bonding and valence electrons using simple conventions:
- Bonds are shown as lines.
- Lone electron pairs (if any) are shown as dots.
- Atom connectivity is drawn in flat space.
Instead of memorizing these conventions right away, we will unpack them from the 3D models. You can see, for instance, that in every structure above, carbon appears bonded to four other atoms. Lewis structures encode that information with four lines extending from each carbon.
Activity: Hydrogen and carbon connections
Look closely at the three molecular models above.
- Where are the carbon atoms located in each structure? How are they connected to one another?
- Where are the hydrogen atoms located, and how many are bonded to each carbon?
- What patterns do you notice in how carbon and hydrogen connect across these three molecules?
Try sketching a Lewis structure for each molecule that reflects these patterns.
Write in your notebook, then left-click here for an explanation.
Let’s take a closer look at these three molecular models — methane (CH4), ethane (C2H6), and propane (C3H8) — to identify patterns that help us sketch Lewis structures. (In the models above, you can left click and drag to rotate the models to view all sides of the molecules.)
First, notice the locations and roles of each type of atom. The carbon atoms are the larger, darker spheres, and they tend to form the core or interior of the molecule. In contrast, the hydrogen atoms are smaller, lighter spheres that appear on the outside or periphery of the structure.
In methane (CH4), there’s a single carbon atom in the center, with four hydrogen atoms bonded around it, each forming one connection.
In ethane (C2H6), we see two carbon atoms connected to each other, forming the start of a carbon chain. Each carbon is also bonded to three hydrogen atoms, which are located around the edges of the molecule. The carbon–carbon bond is in the middle; the hydrogen atoms radiate out from each carbon.
In propane (C3H8), the carbon chain lengthens to three carbon atoms connected in a row. The end carbon atoms each bond to three hydrogens, while the central carbon bonds to two hydrogens and the two neighboring carbons. Again, the hydrogen atoms appear at the ends and edges, while the carbon atoms form a continuous backbone or central chain.
Across all three molecules, we see consistent patterns:
- Each carbon forms four bonds total—either to other carbons or to hydrogens.
- Each hydrogen forms just one bond, always to a carbon.
- Carbon atoms form the interior framework of the molecule, while hydrogens decorate the outside.
When you draw Lewis structures, you will use lines to represent bonds, so you will want to preserve this connectivity. The carbon chain should be drawn as a continuous series of atoms, with hydrogens extending outward from each one. While Lewis structures flatten the molecule into two dimensions, they are meant to reflect the same bonding relationships and connectivity we observe in these space-filling models. See below for the Lewis structure drawings.
3D space-filling models are great for visualizing how atoms occupy space and how they’re arranged. But they’re not so great for drawing on a whiteboard, annotating with electron counts, or quickly comparing two different molecules. That’s why chemists developed Lewis structures. Let’s go back to our example molecules: methane, ethane, and propane. If we translate their 3D structures into 2D Lewis structures, we notice some clear conventions:
- Each carbon makes four lines (bonds).
- Each hydrogen connects with one line (bond).
- We show electrons only when they’re not in bonds.
These are not arbitrary rules—they’re patterns that emerge from the molecular models themselves. Lewis structures don’t create the structure; they reflect it.
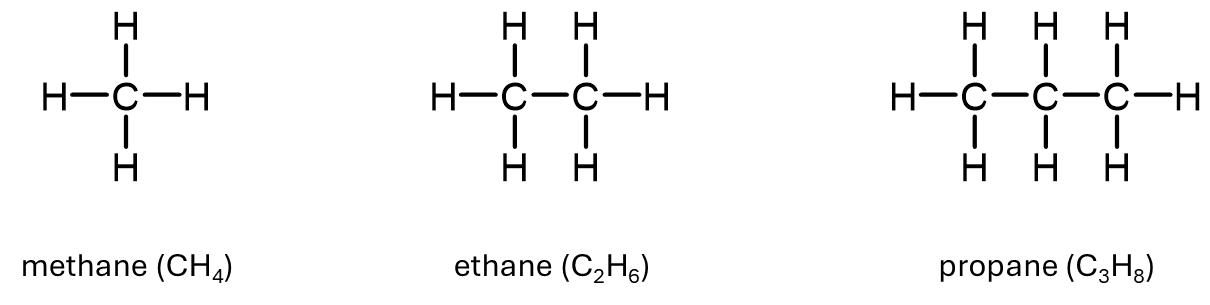
Activity: Hydrocarbon Lewis structures
Examine the Lewis structures for methane, ethane, and propane. How does each one encode the bonding patterns you saw in the 3D models? What is simplified or abstracted in the Lewis version?
Write in your notebook, then left-click here for an explanation.
When we look at the Lewis structures of methane, ethane, and propane, we can see that each one preserves the fundamental bonding pattern seen in the space-filling models: carbon atoms form chains or central frameworks, and each carbon is connected to enough hydrogen atoms to form a total of four bonds. This reflects the typical bonding behavior of carbon (four covalent bonds) and hydrogen (one covalent bond).
However, Lewis structures are simplified in several ways. First, they flatten the molecule into two dimensions, so we lose information about 3D shape (such as the spacing of the hydrogen atoms around the carbon atoms) . Second, the actual sizes of atoms in the real molecule are not represented. In a space-filling model, the smaller hydrogen atoms appear tucked around the outside of the molecule, but in a Lewis structure, they’re just drawn evenly around each carbon for clarity.
Another abstraction is that Lewis structures do not show any variation in bond length. As we will see in Unit 2, bond lengths vary depending on the atoms involved. But in a Lewis structure, all single bonds look the same.
So while Lewis structures do a great job representing how atoms are connected and how valence electrons are distributed, they are not physical models: they’re schematic representations. Chemists use them because they’re easy to draw and annotate, and they support reasoning about electron distribution, charge, and reactivity, even though they simplify the molecule’s real shape and size.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

