D39.1 Lewis Acid-Base Reactions
In the preceding sections, we focused mainly on acid-base reactions within the Brønsted-Lowry definition. These acid-base reactions typically have low activation energies, and both the forward and reverse reactions have a large rate constant. Equilibrium is quickly established when the reactants are mixed.
If we expand the definition to the Lewis definition, which is centered on the electron exchanges that occur in the reaction, then a wider array of molecules can be an acid or a base. Moreover, there is now a much greater variety of acid-base reactions, some of which have relatively slow rates and do not reach equilibrium readily. Let us explore these reactions in a little more detail.
A Lewis acid is a chemical species that can accept a pair of electrons. Another name for it is electrophile (electron-loving). Similarly, a Lewis base, a chemical species that can donate a pair of electrons, is also called a nucleophile (nucleus-loving).
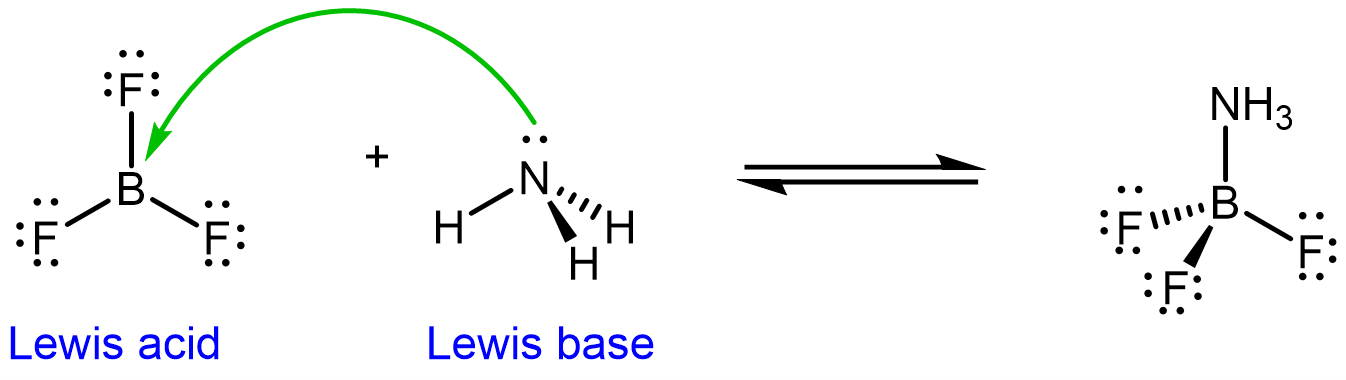
In the above example, BF3 is an electrophile and NH3 is a nucleophile.
Most electrophiles are either positively charged, have an atom that carries a partial positive charge (e.g. the H atom in HCl has a fairly significant δ+ charge), or have an atom that is deficient of an octet (e.g. the B atom in BF3). In other words, either the entire molecule or a part of the molecule is deficient of electron densities. In terms of the Brønsted-Lowry acids and bases we have discussed thus far, stronger acids are usually stronger electrophiles.
Most nucleophiles are either negatively charged (e.g. F¯), have a not-conjugated lone pair (e.g. the N atom in NH3) or a not-conjugated π bond. In other words, either the entire molecule or a part of the molecule has an excess of electron densities. In terms of the Brønsted-Lowry acids and bases we have discussed thus far, stronger bases are usually stronger nucleophiles.
In short, a Lewis acid-base reaction is a reaction where an electron-rich center on a molecule reacts with an electron-poor center on another molecule. The green curved arrow in the example above indicates which is the nucleophile (where the tailend originates from) and which is the electrophile (where the arrowhead is pointing).
Activity: Curved Arrow
For the following reaction:
- First, draw the missing lone pairs on each reactant and product molecules.
- Then, draw the curved arrows on the reactant-side that correctly depict the electron movements that would lead to the formation of the product molecules.
- Finally, indicate which of the reactant is the electrophile, and which is the nucleophile.
(After you have drawn in your notebook, move the slider to compare.)
For example, alkene functional group can undergo addition reactions. The C=C double bond is electron rich: it contains four electrons and the two electrons in the π bonding orbital are higher in energy than those in the σ bonding orbitals. In other words, alkenes can act as a nucleophile.
An alkene can react with a strong acid, such as HBr. In the first step of the reaction, the alkene, acting as the Lewis base, donates a pair of electrons to HBr, an acid in both Brønsted-Lowry and Lewis definition, forming a new C-H σ bond while the H-Br bond breaks forming a bromide anion.
The carbocation formed is a very unstable species (in terms of acidity, it’s a much stronger acid than even the strong acid HBr), and it will readily react as an electrophile (Lewis acid) with the nucleophile (Lewis base) Br¯, to produce the final product of the reaction.
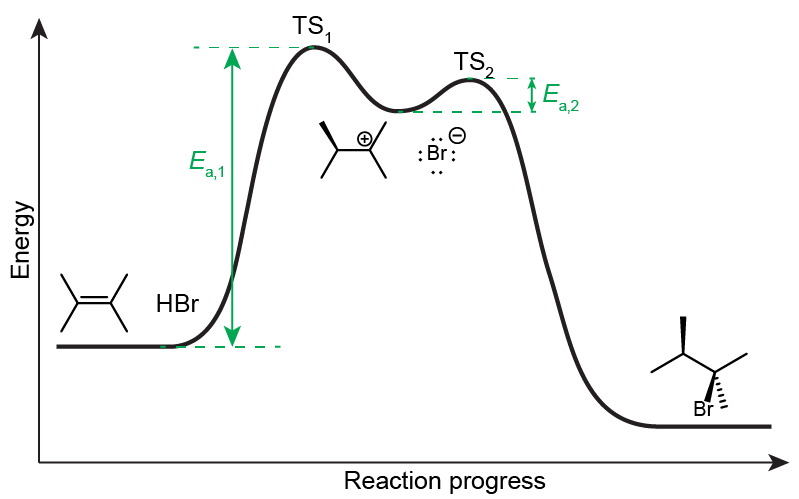
The first step’s activation energy (Ea,1) is much larger than the second step’s activation energy (Ea,2), making step 1 the slow step in the reaction mechanism, and therefore the rate-determining step. The energy required for step 1 is mostly dictated by the reactivity (i.e. the acidity) of the high-energy carbocation. Structural parameters that can stabilize the carbocation (factors that can spread out electron densities and delocalize the positive charge) will lower Ea,1 and increase the rate of the rate-determining step, and therefore dictate the final product of the reaction.
Both reaction steps in the above example are Lewis acid-base reactions, and such reactions are found in many chemical synthesis mechanisms.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

