D6.5 MOs for Heteronuclear Diatomic Molecules
Diatomic molecules with two non-identical atoms are called heteronuclear diatomic molecules. Examples include CO, NO and HCl. Molecular orbital diagrams for these molecules have one more layer of complexity, but they also serve to explain many bond and molecular properties we will encounter later on in the course.
Let’s consider CO as an example. The valence orbitals in both atoms are the 2s and 2p orbitals, but the atomic orbitals in a carbon atom do not have the same energies as those in an oxygen atom. So how do we place these atomic orbitals relative to each other in a MO diagram? We can use their effective nuclear charge to guide us: the effective nuclear charge of oxygen is higher than carbon, which tells us that the oxygen atom’s atomic orbitals are lower in energy than the carbon atom’s. See figure below.
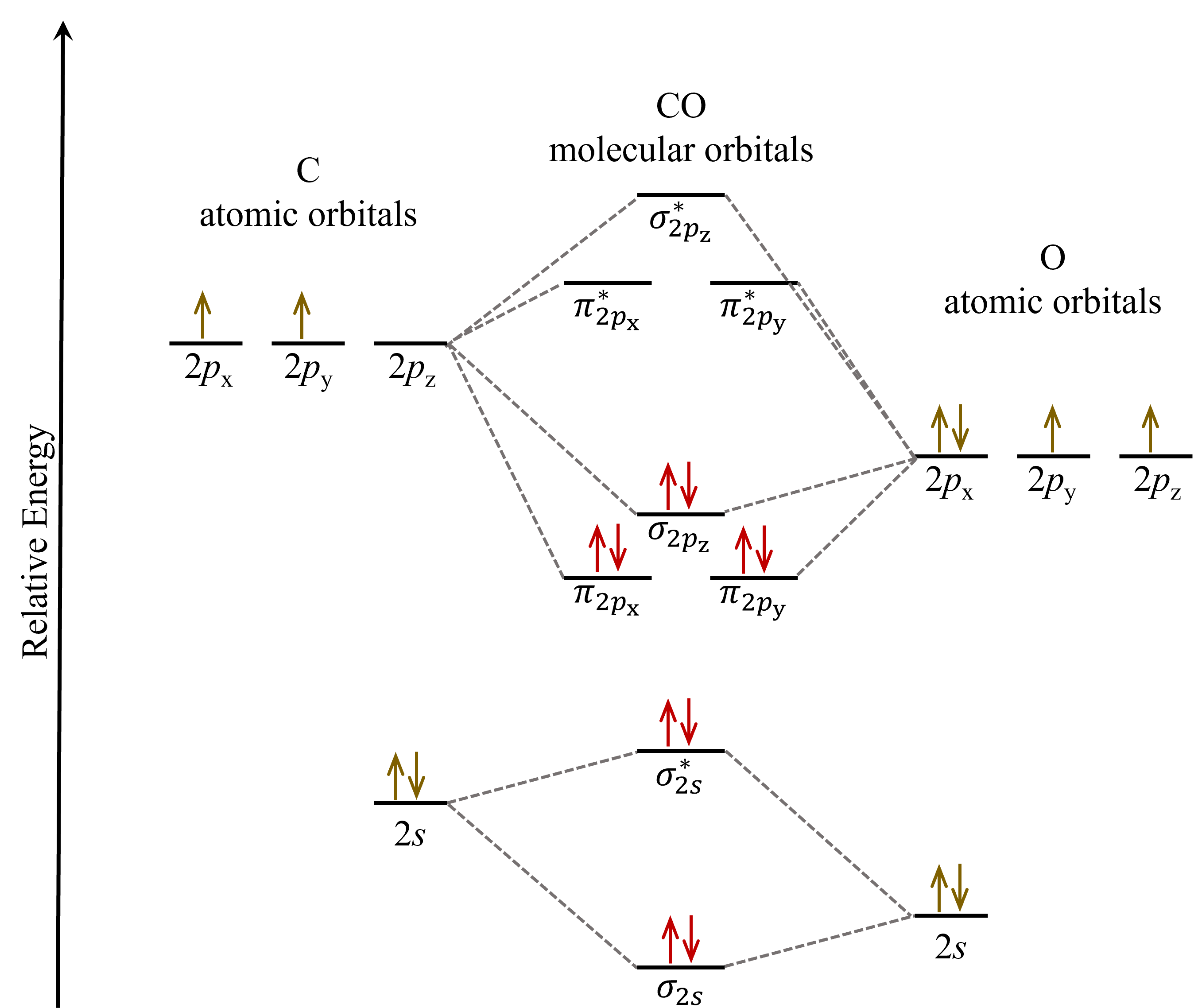
The CO molecular orbital diagram looks skewed compared to that of F2 because the atomic orbitals from the two different atoms do not have the same energies. Therefore, some CO molecular orbitals are energetically closer to the O atomic orbitals while others are closer to the C atomic orbitals, and this leads to asymmetry in the shape of the molecular orbitals. To a first approximation, a molecular orbital is more like an atomic orbital closer in energy to it. For instance, the π2px molecular orbital (left panel in the figure below), which energetically is closer to the oxygen 2px atomic orbital, shows greater density on the oxygen atom. In other words, the π2px molecular orbital has more oxygen 2px character than carbon 2px character.
In comparison, the π*2px molecular orbital, which energetically is closer to the carbon 2px atomic orbital, shows greater density on the carbon atom as it has more carbon 2px character.
When the MO diagram of CO is filled in with the valence electrons, it shows that for a ground state CO molecule, many of the occupied molecular orbitals are energetically closer to the oxygen atomic orbitals. This implies that the bonding electrons in CO are not shared equally between carbon and oxygen atoms. Such unequal sharing of electrons will affect molecular properties such as polarity, a topic into which we will delve more deeply in Unit 2.
Now, let us consider another example, LiF, whose the MO diagram is:
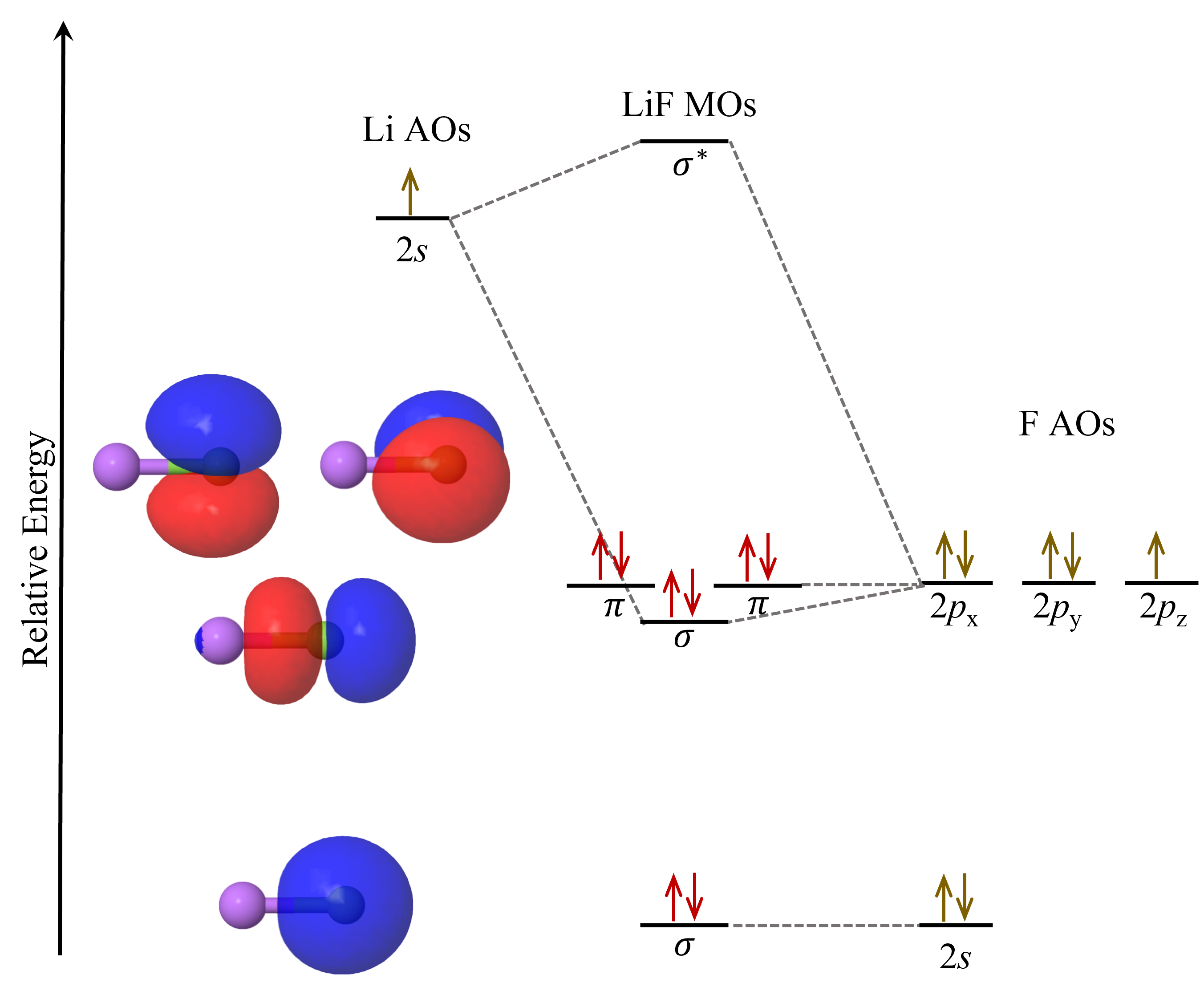
The 2s atomic orbital in Li overlaps with the 2pz atomic orbital in F to form the σ and σ* molecular orbitals. Because of the large energy difference between these two atomic orbitals, the σ molecular orbital lies energetically quite close to fluorine’s 2p atomic orbitals. The boundary-surface plot of the σ molecular orbital also shows that it is quite similar in appearance to the 2pz atomic orbital, and the electron density is largely localized on the F atom.
The 2s, 2px and 2py atomic orbitals in F contribute to neither bonding nor antibonding character. They are called nonbonding molecular orbitals. Their boundary-surface plots show that they are largely unchanged from the atomic orbitals.
The MO diagram for LiF shows that the eight valence electrons in LiF occupy molecular orbitals that, in energy and shape, closely resemble F atom’s 2s and 2p atomic orbitals. That is, most of the electric charge of the valence electrons is on the F atom, making it an F− ion. Li is left with its two core electrons, making it essentially a Li+ ion. This agrees quite well with the ionic bonding model we have previously used to describe LiF, where the bonding interaction is simplified to Coulombic attraction between Li+ and F–. This illustrates the fact that molecular orbital theory can be used to describe bonding interactions in all compounds, including ionic, metallic, and covalent compounds. Molecular orbital theory is more nuanced and includes all the different types of possible interactions.
However, applying molecular orbital theory is not trivial and requires significant computations even for diatomic species. So we often use simpler models for quick and qualitative considerations. For example, when we use the ionic bonding model, we can quickly estimate the relative ionic bond strengths based only on the charges and sizes of ions involved in the ionic compounds.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

