D10.1 New Atoms, Familiar Rules
Although O, N, and the halogens introduce new bonding patterns, the core principles you have practiced still apply:
- Atoms bring a known number of valence electrons, based on their position in the periodic table.
- You count the total number of valence electrons and distribute them to satisfy bonding preferences and (where possible) the octet rule.
- You use Lewis structures to represent both bonding pairs and lone pairs of electrons.
- Formal charge helps evaluate whether a structure is plausible—even when the overall molecule is neutral.
Just as carbon typically forms four bonds and hydrogen one, we often observe other common patterns:
| Atom | Typical # of Bonds | Typical # of Lone Pairs |
| C (carbon) | 4 | 0 |
| N (nitrogen) | 3 | 1 |
| O (oxygen) | 2 | 2 |
| F, Cl, Br, I (halogens) | 1 | 3 |
| H (hydrogen) | 1 | 0 |
These patterns reflect the number of valence electrons each atom brings and how many it needs to share to (typically) reach an octet (or duet for H). But these are not rigid rules. Like we saw with charged hydrocarbons, real molecules sometimes violate these patterns. Still, they’re a valuable starting point, especially when constructing or evaluating Lewis structures for neutral, stable species.
Important: Atoms in the second period (like C, N, and O) cannot exceed the octet in valid Lewis structures. They may have fewer than eight electrons (especially by necessity in ions or radicals), but they cannot have more. The 2s and 2p orbitals available to a second period atom can only house eight electrons.
Even though Lewis structures are two-dimensional, they often reflect trends we observe in experimental data, especially bond lengths. In general, bonds involving smaller atoms (like N or O) are shorter, and larger atoms (like Cl or I) form longer bonds. Also, multiple bonds are shorter and stronger than single bonds. For example, a C≡N triple bond is about 1.1 Å, while a C–C single bond is closer to 1.5 Å. So when you’re building or evaluating Lewis structures, keep in mind that the number and type of bonds has implications for real, measurable properties.
Formal Charge Revisited
Formal charge continues to be a powerful tool as we move beyond hydrocarbons. It helps us evaluate whether the electron distribution in a Lewis structure is consistent with what atoms “prefer” based on their usual valence. Remember, it’s calculated by comparing the number of electrons an atom owns in a structure to its usual number of valence electrons.
But formal charge does not tell us where the electrons actually are. Take carbon monoxide (CO) as an example. The most common Lewis structure places a triple bond between carbon and oxygen, a lone pair on each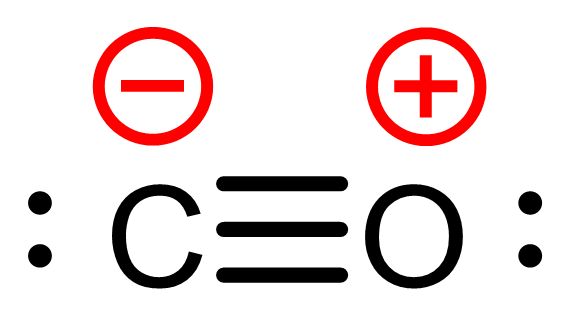 atom, and formal charges of -1 on carbon and +1 on oxygen. That matches the correct number of valence electrons. But it does not reflect the real charge distribution in the molecule.
atom, and formal charges of -1 on carbon and +1 on oxygen. That matches the correct number of valence electrons. But it does not reflect the real charge distribution in the molecule.
Formal charge is a bookkeeping device. It helps us keep track of valence electrons and assess structure plausibility, but it does not always reflect actual electron distribution or chemical polarity—a point we’ll revisit later today. That said, structures with fewer/smaller formal charges tend to be lower in energy and more stable. So even though formal charge is not “real,” it can still help guide us toward better models.
These tools also extend to cyclic compounds—molecules where atoms connect in rings. In these structures, each atom still follows the same basic bonding preferences, but their closed-loop arrangement can create unique effects, such as angle strain or unusual reactivity. We’ll explore examples of cyclic molecules later in this unit, applying the same principles of valence electron counting, octet adherence, and formal charge.
Activity
Below are six chemical species. Some follow the typical bonding patterns outlined in the table above; others deviate. Your task is to analyze them using Lewis structures and reasoning tools.
CH3OH, NH4+, CO, NO2¯, HCN
- Draw a complete Lewis structure for each species, including all bonds and lone pairs.
- For each atom, identify: Number of bonds, Number of lone pairs
- Compare these to the typical bonding patterns (e.g., O: 2 bonds + 2 lone pairs; N: 3 bonds + 1 lone pair), and determine whether each atom follows its typical bonding pattern or not.
- Evaluate the plausibility of the structure: Does each atom obey the octet rule (if in the second period)? What is the formal charge on each atom? Is the structure likely to represent a stable molecule or a high-energy species?
- Justify your reasoning using a combination of octet adherence, formal charge, and comparisons to familiar stable molecules.
You may be surprised how many “rule-breaking” structures are still chemically meaningful, especially when charges are involved.
Draw and write in your notebook, then left-click here for an explanation.
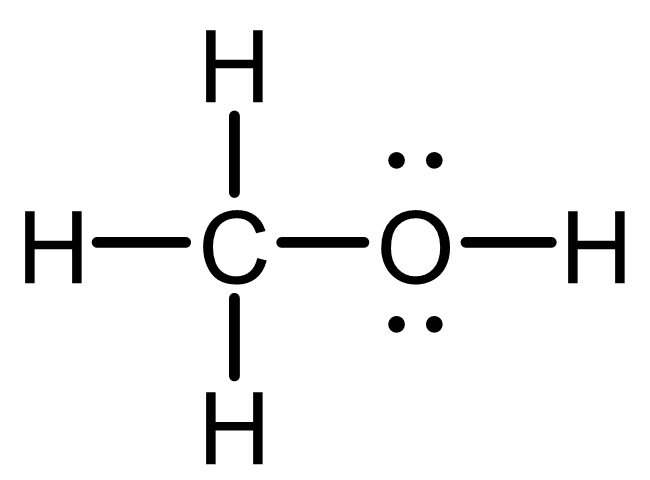 C: 4 bonds, 0 lone pair; typical; obey octet; formal charge=0;
C: 4 bonds, 0 lone pair; typical; obey octet; formal charge=0;
O: 2 bonds, 2 lone pairs; typical; obey octet; formal charge=0;
H: 1 bond, 0 lone pair; typical; obey duet; formal charge=0;
Stable molecule: all atoms obey octet and have 0 formal charge.
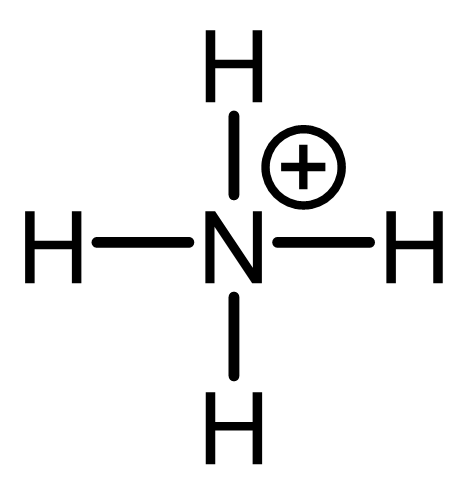 N: 4 bonds, 0 lone pair; not typical; obey octet; formal charge=+1;
N: 4 bonds, 0 lone pair; not typical; obey octet; formal charge=+1;
H: 1 bond, 0 lone pair; typical; obey duet; formal charge=0;
High-energy species: all atoms obey octet but N has +1 formal charge/the molecule is deficient of an electron.
![]() C: 3 bonds, 1 lone pair; not typical; obey octet; formal charge=-1;
C: 3 bonds, 1 lone pair; not typical; obey octet; formal charge=-1;
O: 3 bonds, 1 lone pair; not typical; obey octet; formal charge=+1;
High-energy species: all atoms obey octet but has nonzero formal charges.
![]() N: 3 bonds, 1 lone pair; typical; obey octet; formal charge=0;
N: 3 bonds, 1 lone pair; typical; obey octet; formal charge=0;
one of the O: 2 bonds, 2 lone pairs; typical; obey octet; formal charge=0;
the other O: 1 bond, 3 lone pairs; not typical; obey octet; formal charge=-1;
High-energy species: all atoms obey octet but one O atom has -1 formal charge/the molecule has an excess of electron.
![]() C: 4 bonds, 0 lone pair; typical; obey octet; formal charge=0;
C: 4 bonds, 0 lone pair; typical; obey octet; formal charge=0;
N: 3 bonds, 1 lone pair; typical; obey octet; formal charge=0;
H: 1 bond, 0 lone pair; typical; obey duet; formal charge=0;
Stable molecule: all atoms obey octet and have 0 formal charge.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

