D36.1 pH and Ratio of Conjugate Base to Conjugate Acid
Activity: equilibrium concentrations 1
Typical white vinegar contains 50. g of acetic acid (CH3COOH) per 1.0 L solution, which makes it a 0.83 M aqueous acetic acid solution. The acid-base reaction between acetic acid and water is
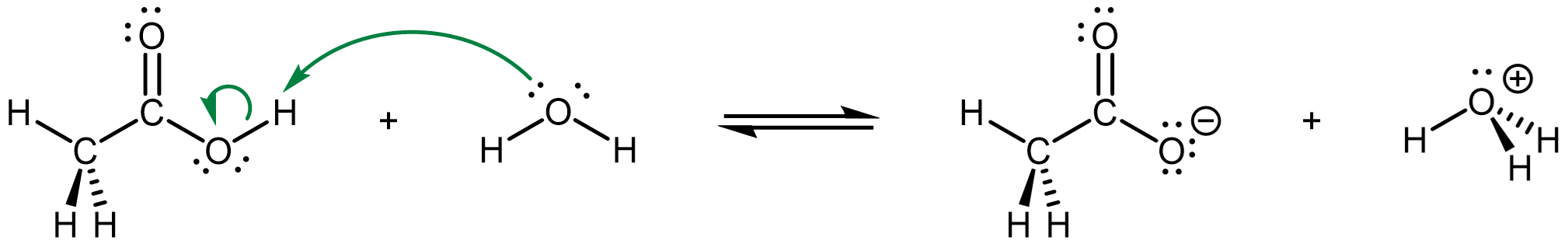
with a Ka of 1.8 × 10-5 at 25 °C.
If you have a beaker of vinegar at 25 °C, how much of each reaction species (CH3COOH, CH3COO¯, H3O+) is present in the solution?
A. First, let's calculate concentration of H3O+. In other words, what is the pH of this 0.83 M acetic acid solution?
Do the work in your notebook, then left-click here for an explanation.
The acid dissociation constant expression for acetic acid is:
[latex]K_a = \dfrac{[\text{CH}_3\text{COO}^{-}]_e[\text{H}_3\text{O}^{+}]_e}{[\text{CH}_3\text{COOH}]_e}[/latex]
Constructing an ICE table gives:
| CH3COOH(aq) | + | H2O(ℓ) | ⇌ | CH3COO¯(aq) | + | H3O+(aq) | |
| Initial concentration (M) | 0.83 | - | 0 | 1.0 × 10-7 | |||
| Change in concentration (M) | -x | - | +x | +x | |||
| Equilibrium concentration (M) | 0.83 - x | - | x | 1.0 × 10-7 + x |
Plugging the equilibrium concentrations into the Ka expression above gives:
[latex]1.8 \times 10^{-5} = \dfrac{(x)(1.0 \times 10^{-7} + x)}{(0.83 - x)}[/latex]
Solving for x gives:
x = 3.8 × 10-3 M
Therefore:
[H3O+] = (1.0 × 10-7 + x) M = ( 1.0 × 10-7 + 3.8 × 10-3) M = 3.8 × 10-3 M
pH = -log ([H3O+]) = -log (3.8 × 10-3) = 2.42
B. The ICE table above also provides information about the concentrations of CH3COOH and CH3COO¯. We can use those values to calculate the percentage of acetic acid existing as the conjugate base (CH3COO¯) in this solution. (This is quite similar to percent dissociation.)
Do the work in your notebook, then left-click here for an explanation.
From the ICE table above:
[CH3COO¯]e = x = 3.8 × 10-3 M
Therefore:
[latex]\%\;\text{CH}_3\text{COO}^{-} = \dfrac{[\text{CH}_3\text{COO}^{-}]_e}{[\text{CH}_3\text{COOH}]_0} \times 100\% = \dfrac{3.8 \times 10^{-3}\;M}{0.83\;M} \times 100\% = 0.46\%[/latex]
0.46% of the acetic acid in this 0.83 M solution exist as the conjugate base giving rise to pH = 2.42.
Imagine if we are able to follow around a single acetic acid molecule in this dynamic equilibrium: it is surrounded by water molecules, forming and breaking hydrogen-bonds as it moves around in the solution. Occasionally, a hydrogen-bond interaction leads to the transfer of a H+ to a water molecule, and the acetic acid becomes an acetate anion. As it continues traversing around in the solution as an acetate, it will encounter a H3O+, leading to a H+ transferring back, and it is again in the acetic acid form. Following this acetic acid molecule around long enough, and we will find it in the CH3COOH form 99.54% of the time, and in the CH3COO¯ form 0.46% of the time.
Therefore, at any given moment in time, we will find 0.46% of the acetic acid in the CH3COO¯ form in this vinegar solution.
Like any other system at equilibrium, when conditions are changed and the system is no longer at equilibrium, the system will react to achieve new equilibrium concentrations.
Activity: equilibrium concentrations 2
Because the concentration of H3O+ is part of this equilibrium, changing the pH of this solution will perturb the equilibrium.
Predict qualitatively what will happen to the solution pH and [CH3COO¯]e when NaOH is added to the solution.
Write in your notebook, then left-click here for an explanation.
The added OH¯ will react with H3O+ present to form H2O, this effectively lowers [H3O+], i.e., the pH of the solution increases when OH¯ is added.
In response to this perturbation, the reaction
CH3COOH(aq) + H2O(ℓ) ⇌ CH3COO¯(aq) + H3O+(aq)
will proceed in the forward direction (towards products) to each a new equilibrium. Therefore, [CH3COO¯]e will increase when OH¯ is added.
If we again imagine following around a single acetic acid molecule: when it is in the CH3COO¯ form, it will encounter H3O+ less frequently because the added OH¯ have reacted away many of the H3O+. Therefore, it will spend more time in the CH3COO¯ form, leading to an equilibrium where there are more CH3COO¯ present at any given moment.
For any acid-base equilibrium, changing the solution pH will change the fraction of acid that exists in its conjugate form (this applies equally to a base and its conjugate acid). For example, we can plot the percentage of acetic acid and acetate anion in a solution as a function of pH:
As the graph above shows, there's a point where the conjugate pair is at a 50:50 ratio. The pH at this point equals the acid's pKa value. This relationship can be derived from the Ka expression:
Rearranging the variables gives:
Taking the negative logarithm of both sides gives:
Therefore, when [HA]e = [A¯]e, [latex]\text{log}\dfrac{[\text{A}^{-}]_e}{[\text{HA}]_e}[/latex] = log(1) = 0, and pH = pKa, as the graph above shows.
This equation can also be rearranged to show that the ratio of conjugate base to conjugate acid is determined by the solution pH:
If a solution's pH is two units above the acid's pKa value, 99% of the acid is in its conjugate base form. Conversely, if the pH is two units below the pKa, 1% of the acid is in its conjugate base form. In other words, raising the solution pH shifts the equilibrium toward the conjugate base and lowering the solution pH shifts the equilibrium toward the acid.
The equation verifies quantitatively the prediction of Le Chatelier's principle that adding strong base would react away hydronium ions, shifting the equilibrium toward products.
Exercise: Acid/base Ratio and pH
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

