D15.7 Condensation Polymers: Polyesters
A condensation polymer is a polymer formed via condensation reactions. A difference between condensation reaction and addition reaction is that, in addition to the main product, there is also a small molecule byproduct, such as H2O, HCl, or some other simple molecule.
Condensation polymers usually grow by forming ester or amide linkages, where new C-O or C-N σ bonds form to link monomers. A typical monomer has a functional group at each end of the molecule. When one end of the monomer reacts and is added onto a polymer chain, the functional group at the other end remains and allows for further reaction to lengthen the polymer chain. Condensation polymerization often, but not always, combine two different monomers in an alternating structure.
Polyester
A polyester is a polymer where the individual units are held together by ester linkages. For example, the common polyester polyethylene terephthalate (abbreviated as PET or PETE; has brand names such as Dacron or Mylar) consists of polymer chains formed from terephthalic acid (benzene-1,4-dicarboxylic acid) and ethylene glycol (ethane-1,2-diol) monomers.
The condensation reaction that results in the formation of the ester linkage in PET is shown below. Repeated condensation reactions result in the step-wise growth of a polymer chain.
The repeating unit of PET is
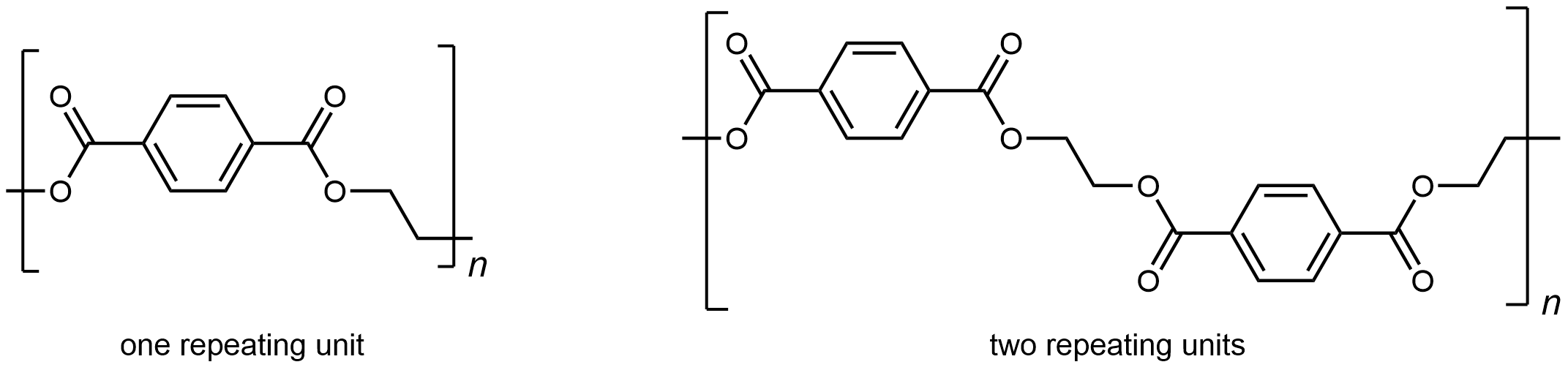
The presence of ester groups, a polar functional group, on the polymer strand increases the intermolecular forces between polymer chains, and thereby increases the crystallinity and tensile strength of the polymer. PET makes for excellent fibers and are used in many fabrics. It is also used to make bottles for soda and other beverages. Polyester is biologically inert, so a knitted polyester tube can be used in surgery to repair or replace diseased sections of blood vessels.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

