D22.2 Predicting Entropy Changes
While calculating exact entropy values requires data, we can predict qualitative trends based on common patterns of energy dispersal.
Phase Changes
As substances move from solid to liquid to gas, their particles gain more freedom of motion, and entropy increases.
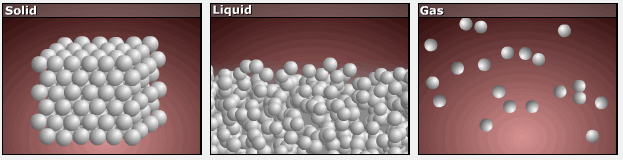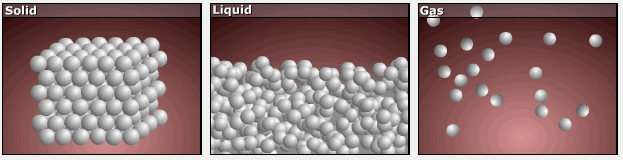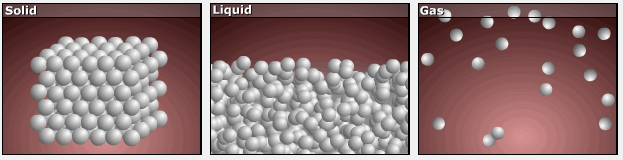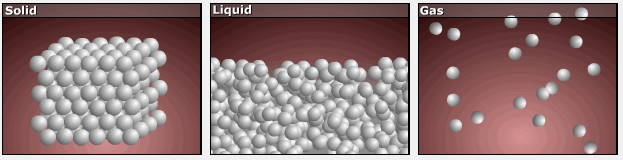
This is reflected in the absolute entropy values seen in the table for the three phases of water in the previous section.
Dissolution
When an ionic compound dissolves in water, it’s tempting to assume that entropy always increases. After all, the solid lattice breaks apart, and the ions spread throughout the solution. This seems like a clear case of energy becoming more dispersed. And in many cases, that is true.
But this is not a universal rule. Some ions (especially small, highly charged ones like Ca²⁺, Mg²⁺, or Al³⁺) form very strong ion–dipole interactions with surrounding water molecules. These interactions create structured hydration shells, where water molecules become tightly oriented around the ion and lose much of their freedom to move or rotate. If the loss of entropy in the water outweighs the gain from dispersing the solute particles, the overall entropy of the system can actually decrease.
Thus, entropy change during dissolution depends on both the dispersal of the solute and the restructuring of the solvent. Think of it as releasing birds from a cage while simultaneously trapping butterflies in (well-ventilated) jars: whether entropy increases or decreases depends on the balance between freedom gained and freedom lost.
| Dissolution Reaction | ΔS° (J/mol·K) | |
| NaCl(s) ⟶ Na+(aq) + Cl¯(aq) | +43 | Entropy increases (ion freedom) |
| Ca(OH)2(s) ⟶ Ca2+(aq) + 2OH¯(aq) | –4 | Entropy decreases (structured water) |
Molecular Complexity and Flexibility
Larger and more flexible molecules typically have higher entropy due to more accessible vibrational and rotational states.
| Molecule | S° (J/mol·K) | |
| Nitrogen, N2 (g) | Diatomic molecule (28 g/mol) | 192 |
| Ethane, C2H6 (g) | Simple, flexible alkane (30 g/mol) | 229 |
| Oxygen, O2 (g) | Diatomic molecule (32 g/mol) | 205 |
| n-Pentane, C5H12 (g) | Longer chain alkane | 348 |
| 2,2-dimethylpropane, C5H12 (g) | Isomer of pentane | 297 |
| Valine C5H11NO2 (s) | Flexible side chain (amino acid) | 179 |
| Proline, C5H9NO2 (s) | Ring structure (amino acid) | 164 |
Changes in Particle Count
Reactions that produce more independently moving species—especially gases—show significant entropy increases, as energy can be distributed across more particles and more translational states. For example,
| Reaction | ΔS° (J/mol·K) | |
| 2NaHCO3(s) ⟶ Na2CO3(s) + CO2(g) + H2O(g) | +145 | Gases produced from solids lead to major increase in entropy |
Each of these examples reinforces a central idea: entropy increases when energy can be spread out more widely—through changes in space, motion, structure, or number of particles.
Using Absolute Entropies to Compute Entropy Changes
Unlike enthalpy, where only changes (ΔH) can be measured, entropy has an absolute scale. That’s because the third law of thermodynamics defines the entropy of a perfect crystal at absolute zero (0 K) as exactly zero. From that reference point, we can measure and tabulate standard molar entropy values (S°) for individual substances. This allows us to compute entropy changes for a reaction by simply subtracting the total entropy of the reactants from the total entropy of the products:
ΔrS° = ∑S°(products) − ∑S°(reactants)
This method works because S° values already reflect the absolute amount of energy dispersal within each substance’s particles at standard conditions.
For example, for the reaction: CaCO3(s) ⟶ CaO(s) + CO2(g), we find from the thermodynamics table in the appendix:
| S° (J/mol·K) | |
| CaCO3(s) | 92.9 |
| CaO(s) | 39.75 |
| CO2(g) | 213.74 |
From which, we can calculate
ΔrS° = (39.75 J/mol·K + 213.74 J/mol·K) – (92.9 J/mol·K) = +160.6 J/mol·K
Entropy increased mostly due to gas formation—energy can now be dispersed over many more translational states.
Exercise: Standard Entropy Change
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

