D7.5 Rings or Double Bonds?
Hydrocarbons follow predictable structure patterns. For example, a straight-chain alkane like butane (C4H10) has no rings or multiple bonds—it’s fully “saturated” with hydrogens.
Now imagine you see a hydrocarbon with fewer hydrogens than expected—say, C4H6 instead of C4H10. That missing hydrogen count is a clue: something must be different about its structure. The molecule may contain:
- A ring, which prevents extra hydrogens from attaching.
- A double or triple bond, which requires extra electron sharing between carbons and replaces places where hydrogens can go.
We call this idea degree of unsaturation. Each time a pair of hydrogen atoms is missing, it points to one structural feature—like a ring or a multiple bond. Therefore, if C4H6 is missing two pairs of hydrogen atoms compared to the saturated version (C4H10), we say it has two degrees of unsaturation, which may mean:
- Two double bonds, or
- One triple bond, or
- A ring and a double bond
Think of the hydrogen shortfall as a signal that something interesting (like a ring or multiple bond) is occuring in the structure.
Activity: degree of unsaturation
The molecular formula C4H6 represents a hydrocarbon with two degrees of unsaturation. Propose at least two possible Lewis structures that account for the missing hydrogens.
- Which of your structures contain a ring?
- Which include multiple bonds?
- How do the structures differ in terms of connectivity between atoms?
Then, for one structure, explain how you know it represents all valence electrons and obeys the octet rule for the carbon atoms.
Draw and Write in your notebook, then left-click here for an explanation.
Contains two rings
![Lewis structure of Bicyclo[1.1.0]butane, C4H6](https://wisc.pb.unizin.org/app/uploads/sites/730/2025/08/bicyclobutane.png)
Contains one ring and one double bond
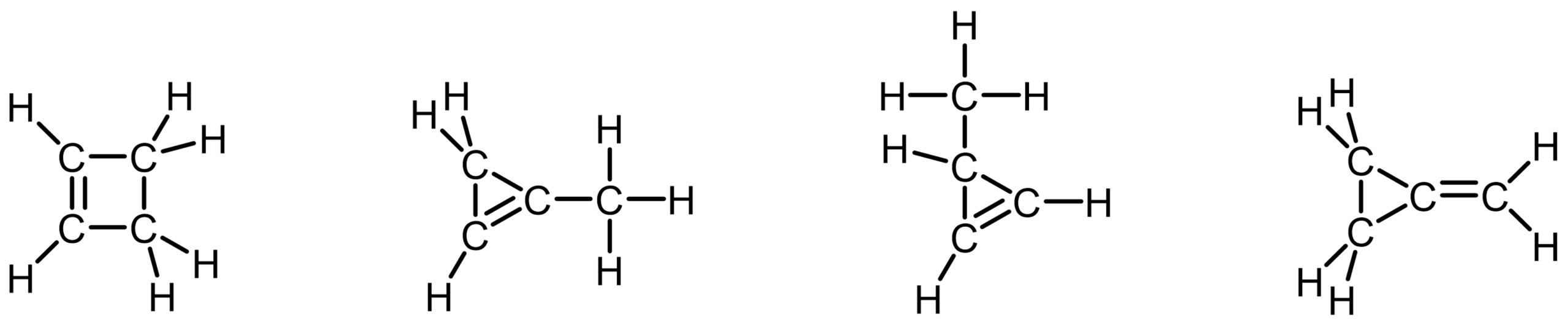
Contains two double bonds
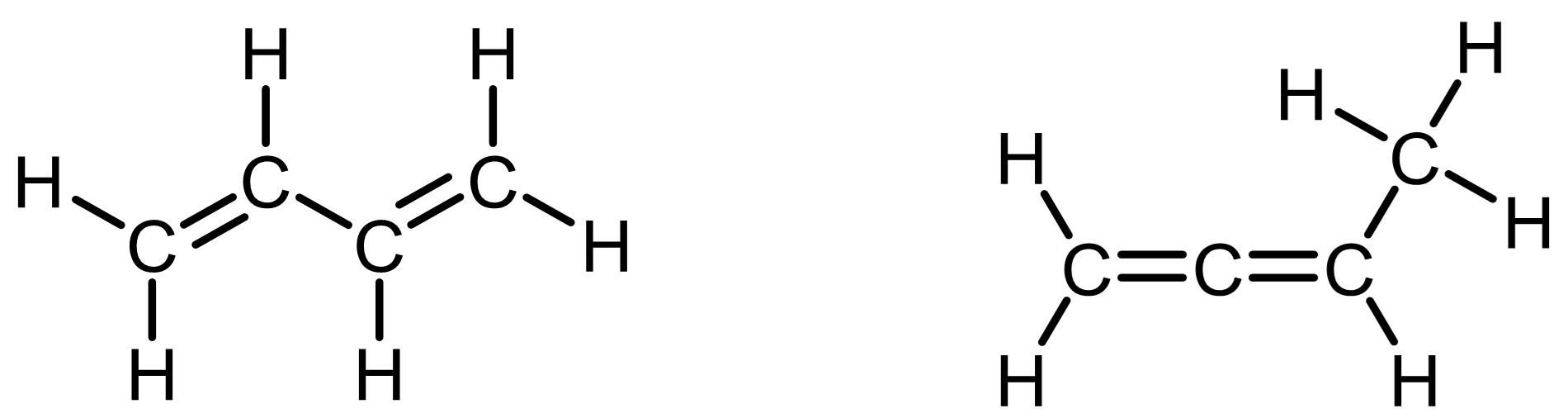
Contains one triple bond

Exercise: Formulas, Multiple Bonds, and Rings
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

