D7.3 Substitution Patterns and Structural Variation
As you move beyond straight-chain hydrocarbons, you’ll notice that some carbon atoms are more “connected” than others. We classify them by substitution level:
- Primary carbon: bonded to one other carbon.
- Secondary carbon: bonded to two other carbons.
- Tertiary carbon: bonded to three other carbons.
- Quaternary carbon: bonded to four other carbons.
For example, the following hydrocarbon molecule shows an example of each type of carbon substitution:
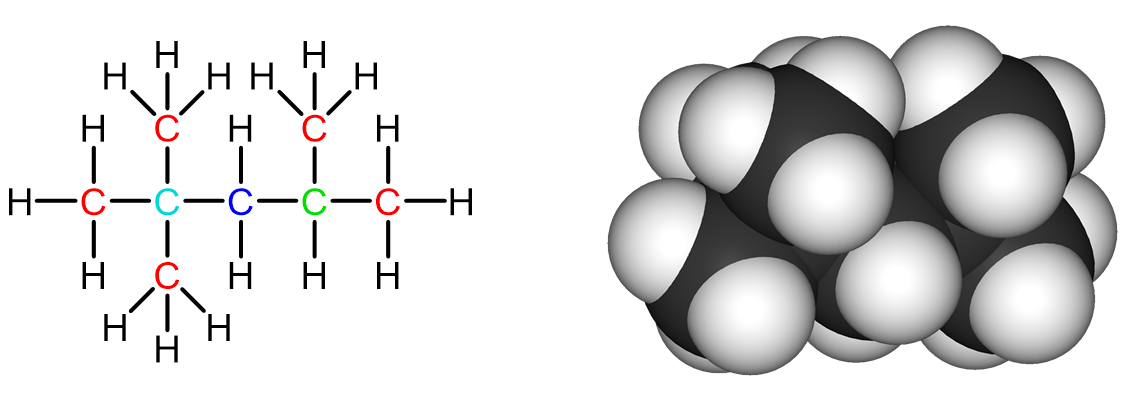
In red are the primary carbons, where each of them are bonded to one other carbon and three hydrogen atoms. They are usually the outermost carbons, found at the ends of the molecule. In blue is the secondary carbon—it is bonded to two other carbons and two hydrogens. In green is the tertiary carbon—it is bonded to three other carbons and one hydrogen. In cyan is the quaternary carbon—it is bonded to four other carbons and no hydrogen.
This classification matters because substitution affects both structure and stability, especially for charged species (see below).
Activity: carbon substitution
Draw two Lewis structures with the formula C4H10 in which the substitution levels of carbon atoms are different. How does this change the molecule’s overall shape?
Draw and Write in your notebook, then left-click here for an explanation.
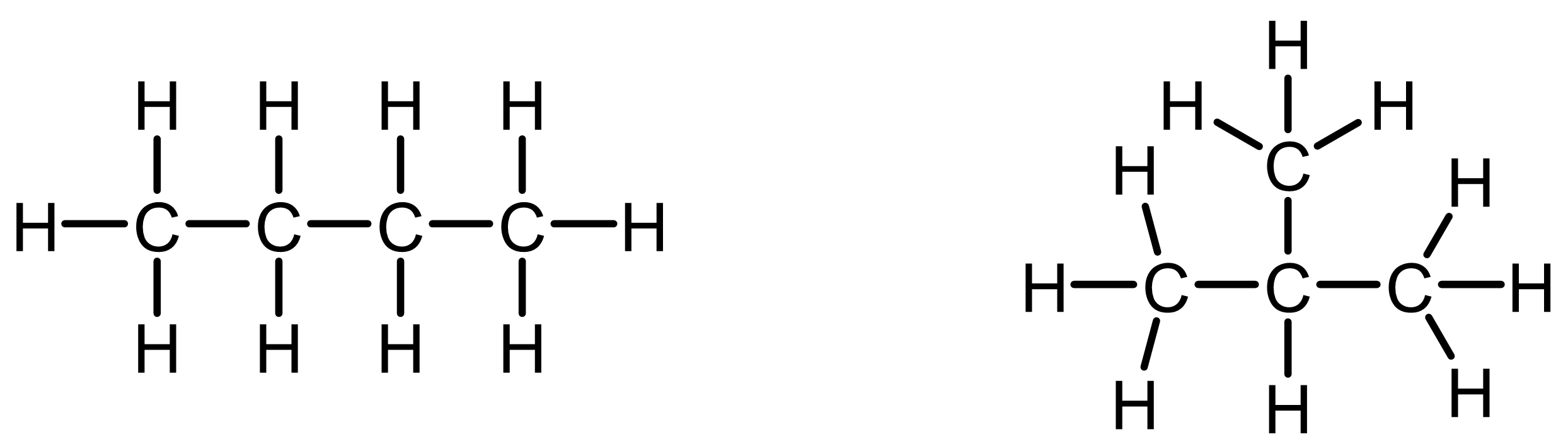
When you draw two versions of a molecule with the formula C4H10, you’ll notice that the way the carbon atoms connect can vary. In one version, the carbon atoms form a continuous chain. In the other, there is a central carbon atom bonded to three other carbon atoms.
This difference in how carbon atoms are connected changes the substitution level of each carbon:
- Some carbons are primary, connected to just one other carbon.
- The left (continuous chain) structure has two primary carbons, one on each end. The right structure has three primary carbons.
- Others are secondary or tertiary, connected to two or three other carbons.
- The left structure has two secondary carbons and no tertiary carbon. The right structure has no secondary carbon and one tertiary carbon.
These substitution patterns affect the overall shape of the molecule. A continuous chain allows for a more extended structure, while a branched pattern results in a shorter, more compact shape (see the space filling models below). As we will see in Unit 2, these structural differences play an important role in determining how the molecule behaves physically and chemically.
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

