Thermochemistry
Potential and Kinetic Energy
Part 1
Read Section 4.1 in your textbook. To use the direct link to the section in your etext provided below, first log into your CengageBrain account and then click the link. http://ng.cengage.com/static/nb/ui/index.html?nbId=72846&nbNodeId=16318991&eISBN=9781285460321#!&parentId=16319126&contentId=JPGAVC889851716
Part 2
For the following scenarios, indicate the which type of potential energy is changing.
Part 3
One aspect of energy that is illustrated in the above set of questions is that energy changes: potential energy can be converted to kinetic energy – and kinetic energy can be converted to potential energy. A roller coaster amusement ride is an example of this. View the animation below to see these energy changes in action.
(Click here to open a larger version of the animation in a new window.)
As the train moves down a hill, potential energy is converted to kinetic energy; as it coasts up a hill, kinetic energy is converted to potential energy.
The next two questions refer to the illustration below.
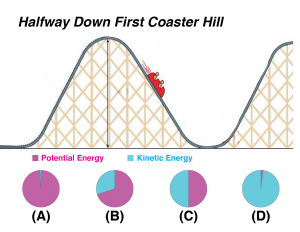
Part 4
Now that we understand how potential and kinetic energy can be converted from one to the other and back again, let’s see how this applies to chemical reactions. (It should be noted that when we are considering potential energy at the level of atoms, ions, and molecules, the predominant type of potential energy is chemical and/or electrostatic potential energy. Both are ways to store energy in chemical bonds. Electrostatic potential energy refers to the energy stored in ionic bonds, such as in a crystal of NaCl. Chemical potential energy refers to the energy stored in covalent bonds, such as in H-O-H. Chemical potential energy also refers to the energy stored in the weaker bonds between molecules, such as in the hydrogen bonds between water molecules.)
Consider the reaction of hydrogen gas combining with oxygen gas to form water:
2 H2(g) + O2(g) → 2 H2O(l)
As you may remember from the in-class demo at the start of the semester, this reaction can be observed by bringing a gas torch in contact with a balloon filled with hydrogen and oxygen gases. When this reaction takes place, a very loud explosion occurs. Below is a chemical potential energy diagram for this reaction. Note the similarities of this diagram and the roller coaster example.
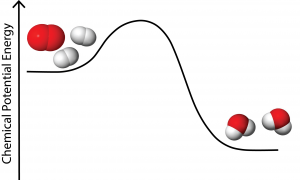
Over the course of the next several class meetings, we will explore how this chemical potential energy can be harnessed in useful ways to power our modern world.

Feedback/Errata