Dissection 02 – Trunk Musculature, Vertebral Column and Spinal Cord
Spinal Cord Syndromes
Ginny Snyder, PhD
Spinal Cord Syndromes
Key Concept: Using knowledge of ascending and descending spinal cord tracts students, will understand how spinal cord injury or disease leads to specific spinal cord syndromes, leading to motor, sensory, reflex and/or autonomic dysfunction.
I. Spinal Cord Injury: Background
Since the spinal cord is the primary pathway of communication between the brain and the PNS, injury or diseases that affect the spinal cord will affect clinical signs and symptoms of the spinal cord. Neurologic deficit related to the spinal cord is referred to as myelopathy. When due to trauma, it is known as (acute) spinal cord injury. When inflammatory, it is known as myelitis. If vascular in nature it is known as vascular myelopathy. One of the most common forms of myelopathy in humans is related to narrowing of the spinal canal (or spinal stenosis) and causes spinal cord compression. This is most commonly caused by osteoarthritis (spondylosis), which results in spinal stenosis and cord compression. This condition most commonly occurs in the cervical spine and is called cervical spondylosis (cervical spondylytic myelopathy).
Spinal cord injury is often characterized as traumatic vs. atraumatic. In traumatic injury, the mechanism of action, including force and direction, is very important in considering how the spinal cord may be injured and the type of deficit that may accompany the injury. Common mechanisms of injury include 1) extremes of motion, 2) axial loading, 3) penetrating, or 4) electrocution. Traumatic injury can lead to disruption of bone, ligaments, intervertebral discs or vascular supply. The injury may result in cord compression, tearing or stretching of ligaments, disc herniation, interruption of blood supply, traction, or penetrating trauma. Atraumatic injury of the spinal cord commonly includes masses (tumors), infection, herniated discs, vascular infarction, autoimmune disorders, inflammation or demyelination and plaque formation (i.e., multiple sclerosis).
Lesions in the spinal cord may result in edema (swelling) and/or hemorrhage, which can cause secondary injury within the spinal cord. The absence of bleeding in the cord is a non-hemorrhagic injury, whereas the presence of bleeding is referred to hemorrhagic injury. Edema and hemorrhage within the cord is identified by imaging (MRI) the cord. Spinal cord bruising is a contusion.
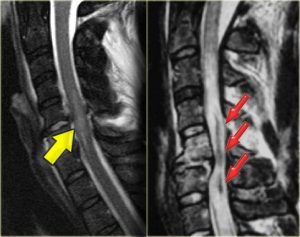
Images: Non-hemorrhagic and hemorrhagic spinal cord injury. The yellow arrow on the left is indicating edema (swelling) inside the spinal cord in response to injury. The red arrows (right) are pointing out a hemorrhage (dark) inside of a large area of edema, which is lighter than the surrounding normal cord (blue arrows). Radiology Assistant. http://rad.desk.nl/images/4912d52076de2spinal-cord1.jpg
There are different ways to classify and refer to spinal cord syndromes. First, a spinal cord deficit is often referred to as the neurologic level and can be further delineated by the sensory level (lowest level of normal sensation), or the motor level. When motor function is affected, paresis is the term to describe weakness, whereas plegia refers to paralysis. Tetraplegia or quadriplegia (injury above C5) involves neurologic loss of all four limbs and the trunk, and depending on the level of the lesion, may affect the muscles of respiration (higher cervical lesions; C1-C2-C3). Paraplegia (injuries at T1 and below) involves the trunk and both lower extremities, resulting from lesions of the thoracic or lumbosacral cord or roots.
Another way to classify spinal cord lesions is to identify whether there is complete or incomplete loss of sensory and motor function below the level of a lesion. A complete lesion of the spinal cord will lead to no sensory or motor function below the level of the lesion, such as transection of the cord. An incomplete lesion will have preservation of some sensory or motor function below the level of the injury.
II. Spinal Cord Injury: Clinical Signs and Symptoms
Neurologic assessment will reveal clinical signs and symptoms of spinal cord lesions. The signs and symptoms may be motor, pain symptoms, sensory signs and symptoms, reflexes, and autonomic dysfunction. Each is described below:
Motor Signs and Symptoms
Lower motor neuron (LMN) signs are found in a limb if some of its muscles are innervated by anterior horn cells (lower motor neurons) affected at the level of the spinal cord lesion. Weakness may be apparent, and muscle atrophy may initially be mild, but over time atrophy may progress. To provide an example, a bilateral spinal cord lesion at the C5 level would injure the anterior horn cells or ventral roots at that level. This would produce LMN signs in the biceps brachii and deltoid muscles (innervated in part by C5). Weakness in these muscles would likely be evident on physical exam. However, muscles innervated by other anterior horn cells/ventral roots at other spinal cord levels would not be affected.
Upper motor neuron (UMN) signs are found in a limb if a spinal cord lesion affects the corticospinal tract(s) in the spinal cord. The bilateral corticospinal tracts are nerve fibers descending to the anterior horn cells which innervate the muscles in the limb. For example, if a bilateral spinal cord lesion at C5 disrupts the corticospinal tracts at that level, it creates UMN signs in both lower extremities, as well as in the bilateral upper extremity musculature innervated by C5 and below. In another scenario, if UMN signs are only evident in the right lower extremity, the corticospinal tract may only be affected by an ipsilateral (right-sided) spinal cord lesion at the cervical or thoracic level, or from a contralateral (left-sided) brain or brainstem lesion (above the decussation of the pyramids in the medulla).
| UMN | LMN | |
| Type of Paralysis
|
Spastic | Flaccid |
| DTR (Deep Tendon Reflex)
|
Hyperreflexia/Brisk | Hyporeflexia/Reduced or Absent |
| Muscle Tone | Hypertonia/Spasticity
|
Hypotonia/Flaccidity |
| Muscle Mass
|
Mild/Disuse Atrophy/Late
(grossly may appear normal) |
Wasting Atrophy/Early
+ Fasciculation |
| Weakness
|
Yes | Yes (Greater) |
| Plantar Reflex (Babinski)/Clonus | + Babinski/Clonus | – Babinski/Clonus |
Pain Symptoms
Radicular (root) pain is a pain described as “shooting, stabbing, electrical shock-like” pain in the dermatomal distribution of a dorsal root. Its presence indicates inflammation of the dorsal nerve root (i.e., Herpes zoster) or may be caused by direct compression to the nerve root (i.e. herniated disc or tumor). Additionally, the presence of the lesion itself may cause a more constant, dull, local or diffuse pain, so different types of pain symptoms can coexist. Analgesics (nerve block) and anti-inflammatory medications (oral or epidural corticosteroid injections) may reduce inflammation or reduce radicular pain.
Sensory Signs and Symptoms
Functionally, a spinal cord lesion in the anterolateral spinothalamic tract on one side creates a pain (tested by pinprick) and temperature deficit in the contralateral body. This is because of the crossing of these fibers at the spinal cord level. The dermatomal deficit only approximates the level of the lesion, since spinothalamic afferent fibers will ascend a couple levels before decussating to the opposite side of the cord. Also, as you know, there is somatotopic localization in the arrangement of the fibers associated with different levels of the cord. As such, a lesion near the center of the spinal cord will potentially disrupt fibers toward the medial aspect of the spinothalamic tract. Since sacral innervation is most lateral (or peripheral), injury to these fibers may be “spared,” and pain and temperature sensation over the sacral dermatomes is preserved. This is called sacral sparing. This indicates there are fibers, and thus dermatomes, preserved. On the other hand, lesions that are external to the cord will commonly compress from the lateral (peripheral) aspect of the cord upon the somatotopic region innervating sacral dermatomes. You have already learned that fasciculus cuneatus has somoatotopic organization such that it supplies the cervical and thoracic region, whereas fasciculus gracilis supplies the lumbar and sacral region of the body. Thus the location or compression of a lesion will determine where the deficit appears on clinical examination. In either case, a spinal cord lesion affecting fasciculus cuneatus and/or fasciculus gracilis on one side may affect position, vibration and discriminative touch in the ipsilateral body, since the dorsal (posterior) column pathways do not decussate in the spinal cord, but rather in the rostral medulla.
Reflexes
You have discussed the anatomy of reflex arcs, with examples. Deep tendon reflexes (DTRs) generally function as protective mechanisms through involuntary responses. Depending on the level and extent of neurological injury, reflexes may or may not be present as outlined below:
- Above T12/L1 reflexes should be present below the level of your injury (this is known as ‘reflexic’)
- At T12/L1 may have some reflexes intact below the level of your injury
- Below T12/L1 will generally have no reflexes below the level of your injury (this is known as ‘flaccid’)
If reflexes are present after a spinal cord injury, an individual will commonly experience involuntary muscle movement to a stimulus. In a non-injured state, initiation of a reflex also sends a signal to the brain to create awareness of what just happened. In spinal cord injury, this message to the brain is blocked, as is the motor response to cancel, correct, or adjust a muscle movement. Instead, the signals are sent back to the motor cells in the spinal cord and cause a reflex muscle spasm. This can result in a twitch, jerk or stiffening of the muscle. Spasm is an exaggerated reflex response to a stimulus. In spinal cord injury, spasm can be helpful to increase circulation to affected muscles (less atrophy), but spasm can also be severe and create contractures and deformities.
Reflexes also play a role in controlling bladder and bowel emptying after spinal cord injury. If a patient has a spinal cord injury, but is ‘reflexic’ (or ‘spastic’), the individual will have some spasm and less atrophy, may be able to trigger some bladder and bowel emptying, and may get reflexic erections. If a patient is ‘flaccid,’ there will be no spasm and more atrophy, loss of bladder tone, flaccid bladder and bowel sphincters, and no reflexic erections.
Autonomic Functions
As you have learned, the small lateral gray horns are present from T1-L2 (sympathetic neurons) and S2-S4 (parasympathetic neurons). Descending fibers involved with visceral and autonomic activities emanate from groups of cells at various levels of the hypothalamus and brainstem. For example, hypothalamic nuclei project to visceral nuclei in both the medulla oblongata and the spinal cord. In the spinal cord these projections terminate upon cell bodies of the thoracic, lumbar, and sacral lateral gray horns (more specifically in the intermediolateral cell columns of the lateral gray horns).

Diagram: Cross section of the spinal cord showing the lateral gray horns (blue) comprised of autonomic (visceral) efferent cell bodies. https://www.studyblue.com
Autonomic dysfunction can be another determinant of site, extent, and severity of spinal cord pathology. In clinical evaluation, this is evaluated largely through bladder and/or bowel continence. Autonomic bladder control is primarily parasympathetic innervation, and thus its function is largely unaffected by lesions to the sympathetic fibers. There is also voluntary innervation of the bladder from ventral horn cells at levels S2-S4. A spinal cord lesion that interrupts descending motor fibers and autonomic fibers above S2 will produce a neurogenic bladder that cannot be emptied voluntary, but will “leak” when it expands beyond capacity. This is called overflow incontinence and is something that spinal-cord injured patients must learn to manage (through intermittent self-catheterization). A similar situation exists with bowel control. Sacral lesions cause a loss of voluntary control and also a loss of the anal reflex, leading to bowel incontinence.
III. Complete Spinal Cord Syndromes
Spinal cord lesions are usually not as straightforward in their presentation as we describe with “hypothetical” lesions or injuries affecting specific spinal cord levels. Multiple levels of spinal cord injury may occur from direct effects of the lesion (from the tumor, hemorrhage, compression) or from indirect (secondary) effects (such as edema, ischemia, inflammation). Also, at any affected spinal cord level, some of the neurons, tracts or roots may be severely affected, whereas others may be mildly affected, and yet, others may be spared. Asymmetry will occur, for example right > left or vice versa.
Having said that, there are a number of spinal cord syndromes that help to clinically localize spinal cord lesions based on the assessment of all spinal cord functions, including motor, reflex, sensory modalities and sphincter function. Spinal cord syndromes are commonly classified as complete or incomplete and can be related to specific pathologies.
The most common complete spinal cord syndrome is called a segmental (transection) syndrome, (also called transverse myelopathy), which encompasses the entire cross-sectional breadth of the spinal cord and affects all functions of the spinal cord at a specific level (neurologic level) or over a few adjacent levels. A total cord transection syndrome results in cessation of function in all ascending and descending tracts and results in the loss of all types of sensation and motor function below the level of the lesion. Transection above C3 will result in cessation of respiration due to loss of the phrenic nerve. Segmental syndrome can happen as a result of an acute traumatic injury, spinal cord hemorrhage, tumor, or transverse myelitis.

Diagram: Different levels of spinal cord injury and extent of tetraplegia, paraplegia. http://nna-leb.gov.lb/files/pictures/1433242049_1432796857barre.jpg
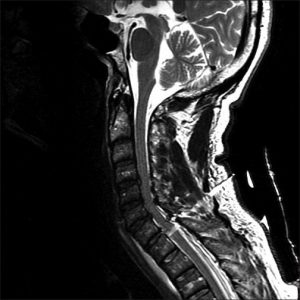
Image: Complete segmental (transection) cord syndrome due to trauma. There is a penetrating course through the overlying soft and bony structures to ultimately transect the spinal cord. http://www.onlinejets.org/articles/2012/5/2/images/JEmergTraumaShock_2012_5_2_204_96503_u1.jpg
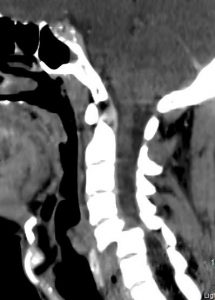
Image: Cervical spine dislocation and spinal cord transection. https://images.search.yahoo.com/search/images
IV. Incomplete Spinal Cord Syndromes
Incomplete spinal cord syndromes are caused by lesions of the ascending or descending spinal tracts that result from trauma, spinal compression, tumor, or hemorrhage/occlusion of spinal arteries. The most common types of spinal cord syndromes include: anterior cord syndrome (ACS), central cord syndrome (CCS), posterior cord syndrome (PCS), Brown-Sequard syndrome, and conus medullaris and cauda equine syndromes. These are described below.
Anterior (or Ventral) Cord Syndrome (ACS)
Anterior cord syndrome (or ACS) involves the anterior 2/3’s of the spinal cord, related to the pattern of blood supply of the anterior spinal artery, which may become occluded or hemorrhage. Since it includes tracts in the anterior 2/3’s of the cord, it affects the ventral and lateral horns, corticospinal tracts, spinothalamic tracts, and also descending autonomic fibers (including sacral centers for bladder control). With bilateral corticospinal tracts being affected, motor weakness or loss will occur, as well as reflexes. However, since this is an UMN lesion affecting the tracts, over time, the effects may include spasticity (hypertonia) and hyperreflexia. Spinothalamic tract deficit creates bilateral loss of pain and temperature sensation. The dorsal pathways are not affected. Urinary incontinence is likely present.
ACS is commonly due to trauma, penetrating injury, [burst] fracture of the vertebrae or as mentioned above, anterior spinal artery occlusion. The prognosis is poor for recovery.

Diagram: Location of lesion in ventral cord syndrome. 2018 UpToDate. https://www.uptodate.com/contents/image?imageKey=NEURO%2F63739&topicKey=NEURO%2F5110&source=outline_link

Diagram: Cross section of the spinal cord demonstrating arterial blood supply. UpToDate 2018. https://www.uptodate.com/contents/image?imageKey=NEURO%2F72176&topicKey=NEURO%2F5110&source=outline_link

Diagram: Anterior spinal artery syndrome. The aqua-colored injury can expand to further affect the lateral corticospinal tracts. https://classconnection.s3.amazonaws.com/654/flashcards/299654/png/screen_shot_2012-10-27_at_115743_pm1351396704053.png
Central Cord Syndrome (CCS)
Central cord syndrome (or CCS) is the most common type of incomplete cord syndrome due to injury in the cervical spine. The injury occurs as a result of anterior and posterior compression (“pinching”) of the spinal cord, leading to edema, hemorrhage, or ischemia to the central portion of the spinal cord. This most commonly occurs in the mid-to-lower cervical cord. As the central lesion enlarges and encroaches upon the corticospinal tracts, due to the somatotopic localization or layering of nerve fibers in the corticospinal tract – with the arm fibers medially and leg fibers laterally – the arms are usually affected more than the legs. Thus, patients typically have more profound motor weakness of the upper extremities (vs. lower extremities).
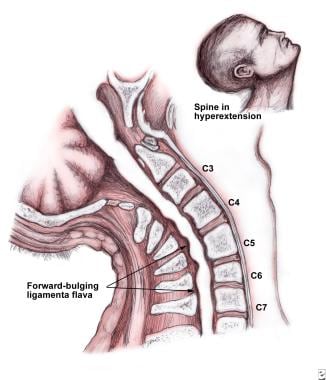


Diagram 1: Hyperextension of the spine in spondolytic patient which may lead to central cord syndrome. This illustrates the pathophysiology of central cord syndrome. Note the “pincher” effect on the central cord by anterior and posterior compression. Image from emedicine.medscape.com. https://emedicine.medscape.com/article/321907-overview
Diagram 2: Sagittal (T2) magnetic resonance imaging (MRI) showing compression of the cord and subsequent edma (presence of white in center of gray cord).
Diagram 3: More substantial angulation and central cord compression leading to impingement and cord edema. Radiopedia. https://radiopaedia.org/articles/traumatic-spinal-cord-injury-1 (far right)
Because the lesion is centrally located, the deficit may also include loss of pain and temperature sensation of one or several adjacent spinal cord levels, caused by disruption at the decussation of spinothalamic tract fibers. Loss of pain and temperature or burning pain in a “cape-like” distribution over the shoulders is classically described. Dorsal column sensations are intact.
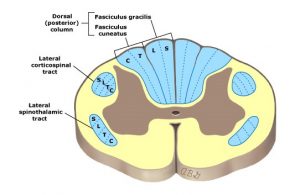


Diagrams (left to right): Major white matter tracts of the spinal cord, demonstrating somatotopic localization (layering), UpToDate 2018 (far left ). American Association of Neurological Surgeons, https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Central-Cord-Syndrome (middle). Cape-like sensory loss to pain and temperature that occurs in a small central cord lesion in the cervical and upper thoracic cord, http://casemed.case.edu/clerkships/neurology/NeurLrngObjectives/Central%20Cord.htm (far right)
CCS is most common in elderly who have significant preexisting cervical spondylosis (osteoarthritis with bone spurs). Due to a fall or motor vehicle accident, hyperextension at the neck, in view of the existing degenerative changes, will lead to anterior/posterior compression. It has a relatively good prognosis with acute injury. Another cause, a spinal cord syrinx (syringomyelia), which is a cavity in the center of the spinal cord, has a more poor prognosis due to the tendency for the syrinx to increase in size over time.
Posterior (or Dorsal) Cord Syndrome (PCS)
Posterior cord syndrome (or PCS) results from bilateral involvement of the dorsal columns, often the corticospinal tracts, and descending autonomic fibers (including sacral centers for bladder control). Dorsal column symptoms will affect ipsilateral deficit of vibration, proprioception, and discriminative touch and will include gait ataxia (lack of voluntary coordination of muscle movementsdue to sensory loss). If the bilateral corticospinal tracts are affected, motor weakness or loss will occur, as well as hyporeflexia; however since this is an UMN lesion affecting the tracts, over time the effects may include spasticity (hypertonia) and hyperreflexia. The spinothalamic pathways are not affected. Urinary incontinence may be present.
Posterior (dorsal) cord syndrome is less common and may occur as a result of trauma or penetrating injury, occlusion of the posterior spinal artery or multiple sclerosis.
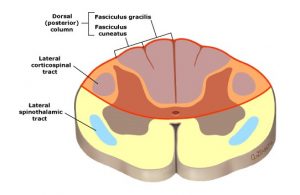
Diagram: Location of lesion in dorsal cord syndrome. UpToDate 2018. https://www.uptodate.com/contents/image?imageKey=NEURO%2F63862&topicKey=NEURO%2F5110&source=outline_link
Brown-Sequard Syndrome
A lesion affecting the right or left half of the spinal cord, a hemisection, is called Brown-Sequard syndrome. Involvement of the spinothalamic tract produces a contralateral deficit to pain and temperature sensation, since they cross at the spinal cord level and ascend on the opposite side of the body. An ipsilateral deficit of vibration, proprioception, and discriminative touch occurs due to involvement of the dorsal (posterior) column pathways, which ascend on the same side of the spinal cord to the medulla, where they decussate to the opposite side. At the same time, with damage to the ventral (anterior) horn cells and corticospinal tract on one side will produce ipsilateral plegia or paralysis, as well as LMN and UMN signs. Since there is unilateral involvement of descending autonomic nerve fibers, urinary continence is usually maintained.
Brown-Sequard syndrome is caused by a hemisection of the cord, commonly due to trauma, tumors, herniated discs, infection, crushing injury or epidural hematoma, and also penetrating (knife, bullet) wounds.
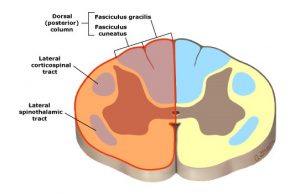
Diagram: Location of lesion in Brown-Sequard syndrome. UpToDate 2018. https://www.uptodate.com/contents/image?imageKey=NEURO%2F58103&topicKey=NEURO%2F5110&source=outline_link
Conus Medullaris Syndrome
The conus medullaris represents the tapering end of the spinal cord at L1-L2. Lesions at (or just above) L2 can compress or constrict the conus medullaris, most commonly due to a large herniated disc, spinal fracture, spinal stenosis, infection, tumor, or penetrating (bullet) wound. Mostly low back pain, with lesser symptoms of bilateral sciatica and/or paresthesia/anesthesia in the lower extremities (as compared to cauda equina). Impotence, difficulty walking, and loss of bowel and bladder function are common. UMN and LMN deficits may occur.
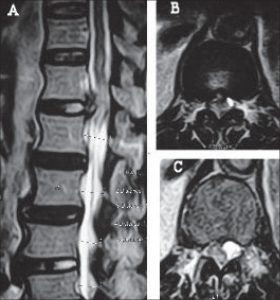
Images: Conus medullartis syndrome on T2 MRI imaging. In sagittal view (A), the red circle shows compression and edema at the tapering end of the spinal cord or conus medullaris. On axial view, diagrams B&C, the conus is being pushed laterally within the spinal canal. The “whiteness” within the cord is again evidence of edema. BioMedSearch.com http://www.biomedsearch.com/nih/Conus-medullaris-syndrome-due-to/19823664.html
Cauda Equina Syndrome
Although not technically a spinal cord syndrome, because it affects the lumbosacral nerve roots rather than the spinal cord itself, it is common to include cauda equina syndrome in this discussion. This occurs in injury to the lumbar spine where the lumbosacral nerve roots of the cauda equina become compressed (usually multiple nerve roots simultaneously). It can be of varying severity and motor fibers are more susceptible than sensory fibers. The patient has concurrent low back pain, often severe, and sciatica. In a significant case of cauda equina, damage to the nerve roots can lead to complete bladder and bowel paralysis. Sensory distribution of cauda equina is characterized by “saddle” anesthesia over the thighs, perineum, and buttocks. If it is a serious cauda equina, damage may be irreversible, but if caught early and the nerve roots are decompressed, there is greater capacity for recovery. This is considered a neurosurgical emergency.
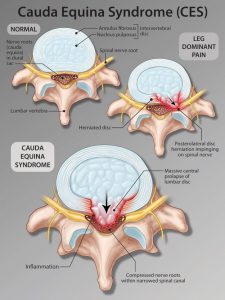
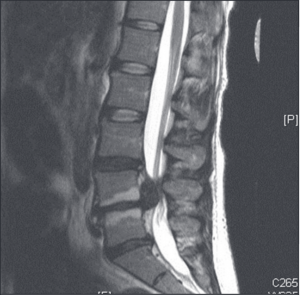
Diagram: Cauda equina syndrome from lumbar disc herniation. https://i.pinimg.com/originals/12/7c/54/127c5430822380f011b443641d15845d.png Image: Sagittal (T2) MRI showing protruding herniated disc compressing cauda equina.
V. Summary
The differential diagnosis of spinal cord injury/disease, or myelopathy, is vast, but can be narrowed down by the clinical syndrome. Yet there are diseases that cause a unique pattern of deficit(s). For example, amyotrophic lateral sclerosis or ALS, progressively destroys UMNs and LMNs, but is very selective. In ALS, sensory pathways are not affected and bowel and bladder functions are normal, but over time there is diffuse weakness with UMN and LMN signs often leading to its diagnosis. Because it only affects motor neurons, it is often referred to as the pure motor syndrome. Another interesting condition is neurosyphilis (tabes dorsalis), which commonly affects the dorsal roots and dorsal spinal cord (as the name implies), producing radicular pain in the lower limbs, impairment of vibration, proprioception, and discriminative touch in the lower limbs and eventually affects all sensations of the lower limbs as the dorsal roots are progressively affected. However, neurosyphilis does not affect the corticospinal tracts and thus strength remains intact. This is considered a dorsal cord syndrome. Understanding the anatomy of the spinal cord and tracts are integral to understanding the pathology of neurologic diseases. For example, just knowing that the anterior spinal artery supplies the anterior 2/3’s of the spinal cord, in an anterior spinal artery occlusion or hemorrhage, you can deduce what tracts are affected and thus the deficits that will present.
These and many more neurological conditions are soon to come in your first module of Clinical Medicine I in fall semester and again in Emergency Medicine in the spring semester (as well as other courses)!
Spinal Cord Syndromes
| Syndrome | Clinical manifestations | Causes |
| Segmental (transection) syndrome | Loss of all sensory modalities, motor loss below affected level; bladder dysfunction |
Trauma, hemorrhage, tumor, epidural abscess, transverse myelitis (inflammatory)
|
| Dorsal cord syndrome | Loss of proprioception, vibratory sensation; variable weakness and bladder dysfunction | Tabes dorsalis, AIDS myelopathy, epidural tumors, cervical spondylotic myelopathy, multiple sclerosis |
| Ventral cord syndrome (anterior spinal artery syndrome) |
Loss of pain and temperature sensation, weakness, bladder dysfunction
|
Spinal cord infarction or anterior spinal artery occlusion, disc herniation, radiation, penetrating injury, [burst] vertebral fracture
|
| Brown Sequard syndrome |
Ipsilateral weakness and loss of proprioception; contralateral loss of pain and temperature sensation |
Knife or bullet injury, multiple sclerosis |
| Central cord syndrome | Segmental loss of pain and temperature, weakness often greater in the arms than legs | Trauma, tumor, acute hyperextension with cervical spondylotic myelopathy, syringomyelia, |
|
Pure motor syndrome
|
Weakness without sensory disturbance | Poliomyelitis, amyotrophic lateral sclerosis (ALS) |
|
Conus medullaris syndrome
|
Bladder and rectal dysfunction, saddle anesthesia | Large disc herniation, trauma, tumor, spinal fracture, lumbar spinal stenosis, infection, or penetrating (bullet) wound |
| Cauda equina syndrome | Radicular pain, leg weakness, and sensory loss; bladder dysfunction |
Large disc herniation, trauma, tumor, lumbar spinal stenosis, infection (i.e., arachnoiditis)
|
2018 UpToDate. https://www.uptodate.com/contents/image?imageKey=NEURO%2F62066&topicKey=NEURO%2F5110&source=outline_link
