10 Hydrocarbons – Alkanes
Learning Objectives
- Draw structures and write chemical formulas for alkanes and cycloalkanes when given only the compound’s name.
| Nomenclature | - Given the molecular formula of an organic compound, draw all of its structural isomers.
| Isomers | - Use strength of intermolecular forces in organic molecules to explain differences in physical properties such as boiling point and solubility.
| Intermolecular Forces |
| Key Concepts and Summary | Glossary | End of Section Exercises |
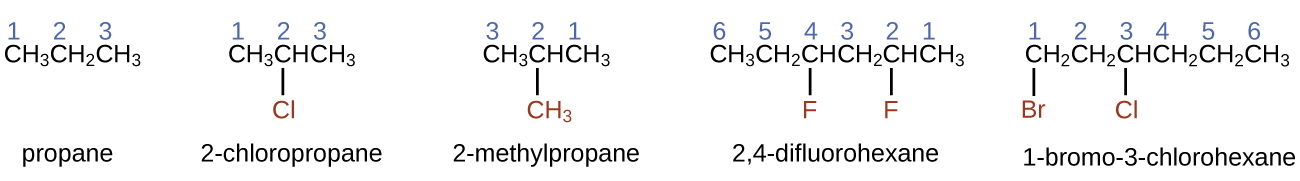
Hydrocarbons
The largest database[1] of organic compounds lists about 10 million substances, which include compounds originating from living organisms and those synthesized by chemists. The number of potential organic compounds has been estimated[2] at 1060—an astronomically high number. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to four strong, covalent bonds to other carbon atoms, resulting in chains and rings of many different sizes, shapes, and complexities.
The simplest organic compounds contain only the elements carbon and hydrogen and are called hydrocarbons. Even though they are composed of only two types of atoms, there is a wide variety of hydrocarbons because they may contain varying lengths of chains, branched chains, rings of carbon atoms, or combinations of these structures. Many hydrocarbons are found in plants, animals, and their fossils; other hydrocarbons have been prepared in the laboratory. We use hydrocarbons every day, mainly as fuels, such as natural gas, acetylene, propane, butane, and components of gasoline, diesel fuel, and heating oil. The familiar plastics polyethylene, polypropylene, and polystyrene are also hydrocarbons. Several classifications of hydrocarbons can be defined by the type(s) of carbon-carbon bonds present. We will discuss several types in the following sections.
Alkanes
Alkanes, or saturated hydrocarbons, contain only single covalent bonds between carbon atoms. Each of the carbon atoms in an alkane has sp3 hybrid orbitals and is bonded to four other atoms, each of which is either carbon or hydrogen. Alkanes all have similar bonds, structures, and formulas; noncyclic alkanes all have a formula of CnH2n+2. The number of carbon atoms present in an alkane has no limit.
Nomenclature: Naming Organic Molecules
Nomenclature is a set of rules to allow for systematic, and consistent, naming of chemical compounds. The International Union of Pure and Applied Chemistry (IUPAC) has devised a system of nomenclature that begins with the names of the alkanes and can be adjusted from there to account for more complicated structures.
The nomenclature for alkanes is based on two rules:
- To name an alkane, first identify the longest chain of carbon atoms in its structure. A two-carbon chain is called ethane; a three-carbon chain, propane; and a four-carbon chain, butane. Longer chains are named as follows: pentane (five-carbon chain), hexane (6), heptane (7), octane (8), nonane (9), and decane (10). These prefixes can be seen in the following table:
Number of Carbons Prefix Number of Carbons Prefix 1 meth- 6 hex- 2 eth- 7 hept- 3 prop- 8 oct- 4 but- 9 non- 5 pent- 10 dec- - Add prefixes to the name of the longest chain to indicate the positions and names of substituents. Substituents are branches or functional groups that replace hydrogen atoms on a chain. The position of a substituent or branch is identified by the number of the carbon atom it is bonded to in the chain. We number the carbon atoms in the chain by counting from the end of the chain nearest the substituents.
- When more than one type of substituent is present, either on the same carbon atom or on different carbon atoms, the substituents are listed alphabetically.
- Because the carbon atom numbering begins at the end closest to a substituent, the longest chain of carbon atoms is numbered in such a way as to produce the lowest numbers for the substituents.
- The ending -o replaces -ide at the end of the name of an electronegative substituent (in ionic compounds, the negatively charged ion ends with -ide like chloride; in organic compounds, such atoms are treated as substituents and the -o ending is used, like chloro).
- The number of substituents of the same type is indicated by the prefixes di- (two), tri- (three), tetra- (four), and so on (for example, trimethyl- indicates three -CH3 substituents bound to the longest carbon chain and difluoro- indicates two fluoride substituents).
- The total amount of numbers for each substituent should match the number of substituents (for example, if you have two fluorine groups, you should have two numbers along with the prefix di-, as seen below).
Alkyl Groups
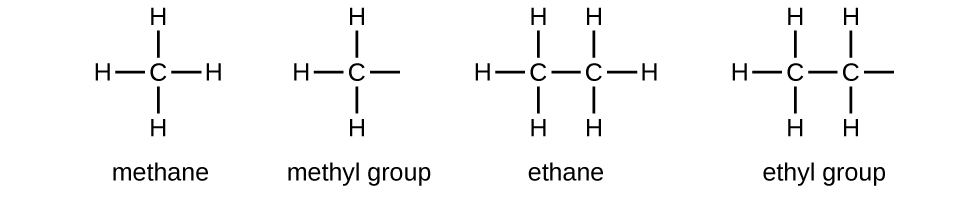
We call a substituent that contains one less hydrogen than the corresponding alkane an alkyl group. The name of an alkyl group is obtained by dropping the suffix -ane of the alkane name and adding -yl:
The open bonds in the methyl and ethyl groups indicate that these alkyl groups are bonded to another atom. The names and structures of these and several other alkyl groups are listed in the following table:
| Alkyl Group | Structure |
| methyl | -CH3 |
| ethyl | -CH2CH3 |
| propyl | -CH2CH2CH3 |
| butyl | -CH2CH2CH2CH3 |
| isopropyl | 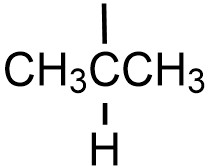 |
| tert-butyl | 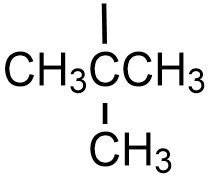 |
Note that alkyl groups do not exist as stable independent entities. They are always a part of some larger molecule.
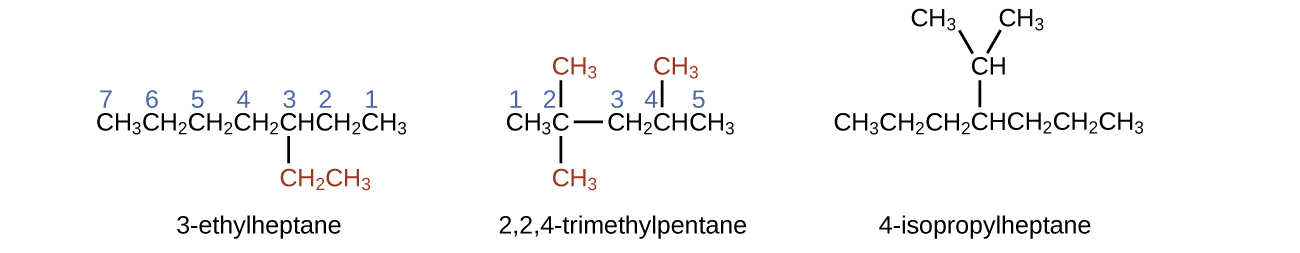
Example 1
Naming Substituted Alkanes
Name the molecule whose structure is shown here:
Solution
- The longest carbon chain runs horizontally across the page and contains six carbon atoms (this makes the base of the name hexane, but we will also need to incorporate the name of the branch).
- In this case, we want to number from right to left (as shown by the blue numbers) so the branch is connected to carbon 3 (imagine the numbers from left to right—this would put the branch on carbon 4, violating our rules).
- The branch attached to position 3 of our chain contains two carbon atoms (numbered in red)—so we take our name for two carbons eth- and attach -yl at the end to signify we are describing a branch.
- Putting all the pieces together, this molecule is 3-ethylhexane.
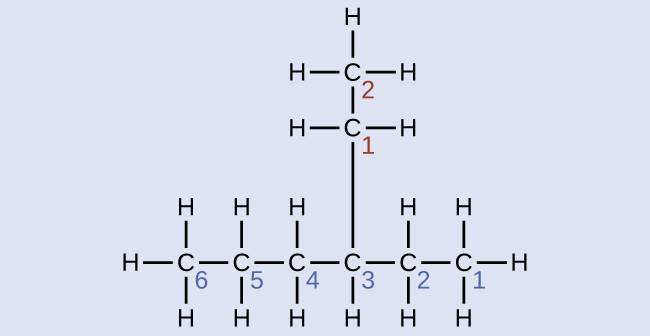
Check Your Learning
Name the following molecule:
Answer:
4-propyloctane
Example 2
Naming Halogen-substituted Alkanes
Name the molecule whose structure is shown here:
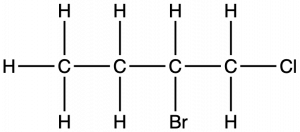
Solution
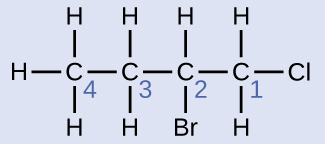
- The four-carbon chain is numbered from the end with the chlorine atom. This puts the substituents on positions 1 and 2 (numbering from the other end would put the substituents on positions 3 and 4).
- Four carbon atoms means that the base name of this compound will be butane.
- The bromine at position 2 will be described by adding 2-bromo-; this will come at the beginning of the name, since bromo- comes before chloro- alphabetically.
- The chlorine at position 1 will be described by adding 1-chloro-, resulting in the name of the molecule being 2-bromo-1-chlorobutane.
Check Your Learning
Name the following molecule:
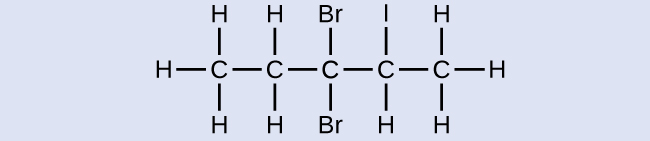
Answer:
3,3-dibromo-2-iodopentane
Isomers
Hydrocarbons with the same formula, including alkanes, can have different structures. For example, two alkanes have the formula C4H10: They are called n-butane and 2-methylpropane (or isobutane), and have the following Lewis structures.)
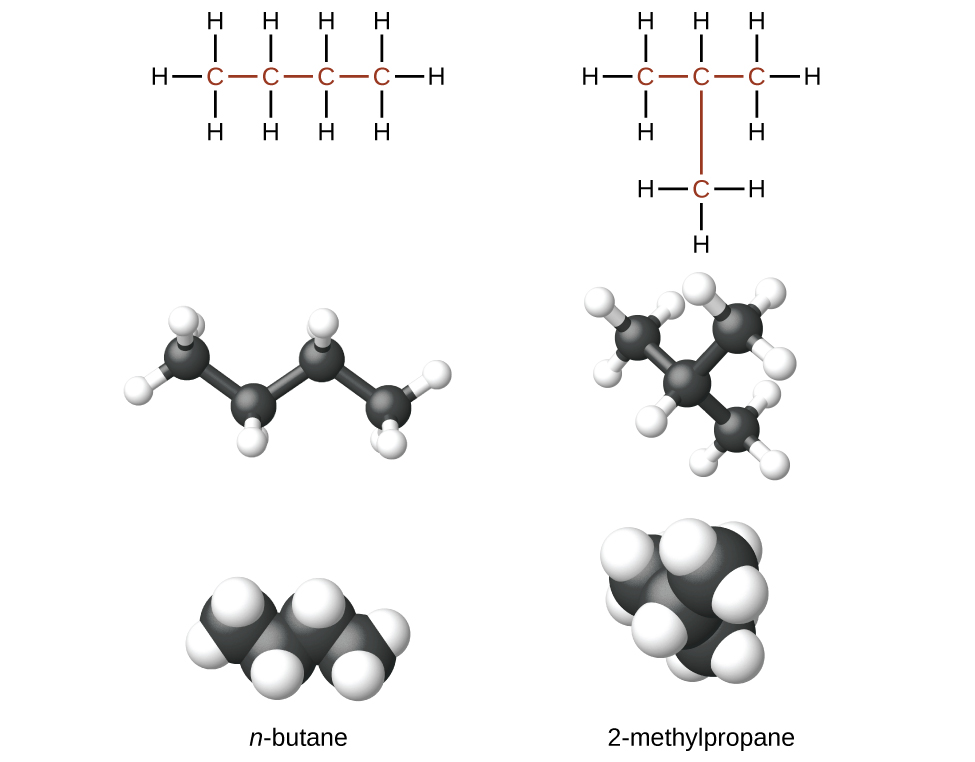
The compounds n-butane and 2-methylpropane are structural isomers (the term constitutional isomers is also commonly used). Structural isomers have the same molecular formula but different spatial arrangements of the atoms in their molecules. The n-butane molecule contains an unbranched chain, meaning that no carbon atom is bonded to more than two other carbon atoms. We commonly use the term normal, or the prefix n, to refer to a chain of carbon atoms without branching. The compound 2–methylpropane has a branched chain (the carbon atom in the center of the Lewis structure is bonded to three other carbon atoms.)
Example 3
Determining Possible Structural Isomers from a chemical formula
Use bond-line structures to indicate all possible structural isomers of heptane, C7H16.
Strategy: An orderly approach can be helpful!
-
- Begin with the longest possible carbon chain.
- Remove one carbon from the longest chain and find unique locations where a -CH3 group may be added to the remaining carbon chain.
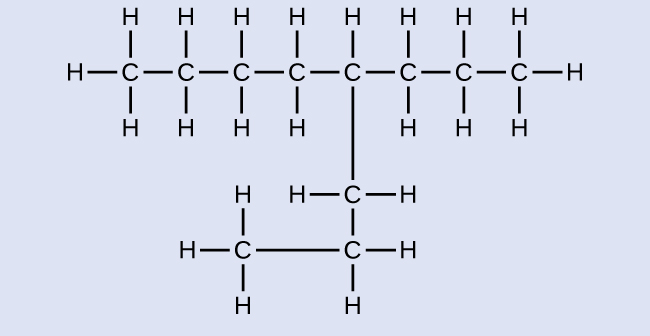
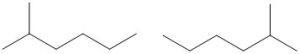
Note: Counting the carbon chain from both sides can help make sure you do not accidentally create identical structures, rather than unique isomers. In the following drawing, the molecules look different, however, counting from both sides indicates the methyl group is on carbon #2 in both molecules and that they are, in fact, the same molecule.
- Repeat by removing an additional carbon from the longest chain and then distributing the carbons in unique locations to form more isomers.
Solution:
| Number of Carbons in the Longest Chain | Isomers |
| 7 | |
| 6 | 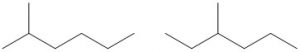 |
| 5 | 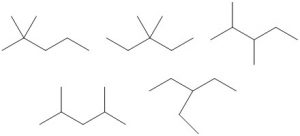 |
| 4 | 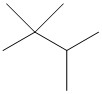 |
Check Your Learning
How many structural isomers of C4H9Cl are there?
Hint: Start by finding the isomers of butane, without the chlorine, and then find unique places to substitute a Cl for a H in the molecules.
Answer:
4
Intermolecular Forces
The low polarity of the carbon hydrogen bonds found in alkanes means that only dispersion forces are found in alkanes. This leads to alkanes having relatively low melting and boiling points and low solubility in polar solvents such as water. Within alkanes, there can be a great deal of variation in the strength of the dispersion forces.
- As the molar mass of the molecules increases, the strength of the dispersion forces increase.
- Increasing the potential surface contact between molecules increases dispersion forces so longer carbons chains will have greater dispersion forces.
- Different isomers of alkanes with the same chemical formula may also exhibit differing strengths of dispersion forces. In general, the less branched an isomer is (or the longer the main carbon chain is), the greater surface area the molecule will have to interact with other molecules, leading to stronger dispersion forces.
The following table demonstrates how increasing the molar mass of straight chain alkanes affects many properties of the compound (STP indicates a temperature of 0 °C and a pressure of 1 atm):
| Alkane | Molecular Formula | Melting Point (°C) | Boiling Point (°C) | Phase at STP | Number of Structural Isomers |
|---|---|---|---|---|---|
| methane | CH4 | –182.5 | –161.5 | gas | 1 |
| ethane | C2H6 | –183.3 | –88.6 | gas | 1 |
| propane | C3H8 | –187.7 | –42.1 | gas | 1 |
| butane | C4H10 | –138.3 | –0.5 | gas | 2 |
| pentane | C5H12 | –129.7 | 36.1 | liquid | 3 |
| hexane | C6H14 | –95.3 | 68.7 | liquid | 5 |
| heptane | C7H16 | –90.6 | 98.4 | liquid | 9 |
| octane | C8H18 | –56.8 | 125.7 | liquid | 18 |
| nonane | C9H20 | –53.6 | 150.8 | liquid | 35 |
| decane | C10H22 | –29.7 | 174.0 | liquid | 75 |
| tetradecane | C14H30 | 5.9 | 253.5 | solid | 1858 |
| octadecane | C18H38 | 28.2 | 316.1 | solid | 60,523 |
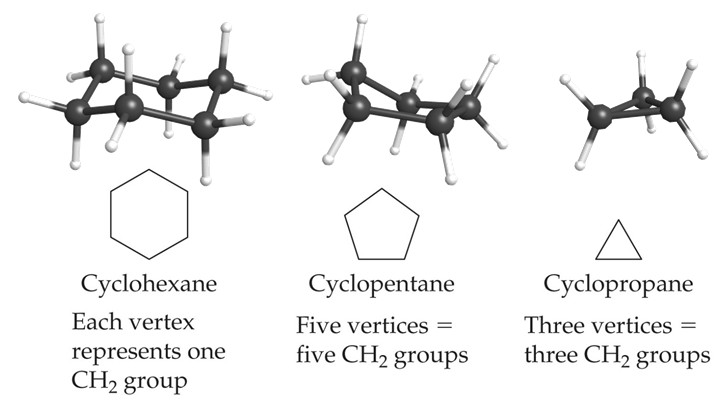
Cycloalkanes
Cycloalkanes are cyclic alkanes where the carbon atoms are connected in a ring. They have a general formula of CnH2n. This differs from the general alkane formula because in joining the carbon atoms into a ring, two hydrogen atoms will have been removed.
They are named in the same manner as alkanes but with the prefix cyclo- added to the name.
Chemistry in Real Life: Combustion
Alkanes are relatively stable molecules, but heat or light can activate reactions that involve the breaking of C–H or C–C single bonds. The combustion of methane is one such reaction:
Alkanes burn in the presence of oxygen, a highly exothermic oxidation-reduction reaction that produces carbon dioxide and water. As a consequence, alkanes are excellent fuels. For example, methane, CH4, is the principal component of natural gas. Butane, C4H10, used in camping stoves and lighters is an alkane. Gasoline is a liquid mixture of continuous- and branched-chain alkanes, each containing from five to nine carbon atoms, plus various additives to improve its performance as a fuel. Kerosene, diesel oil, and fuel oil are primarily mixtures of alkanes with higher molecular masses. The main source of these liquid alkane fuels is crude oil, a complex mixture that is separated by fractional distillation. Fractional distillation takes advantage of the different boiling points for components of the mixture. This is a real life example of how boiling point is a function of intermolecular interactions.
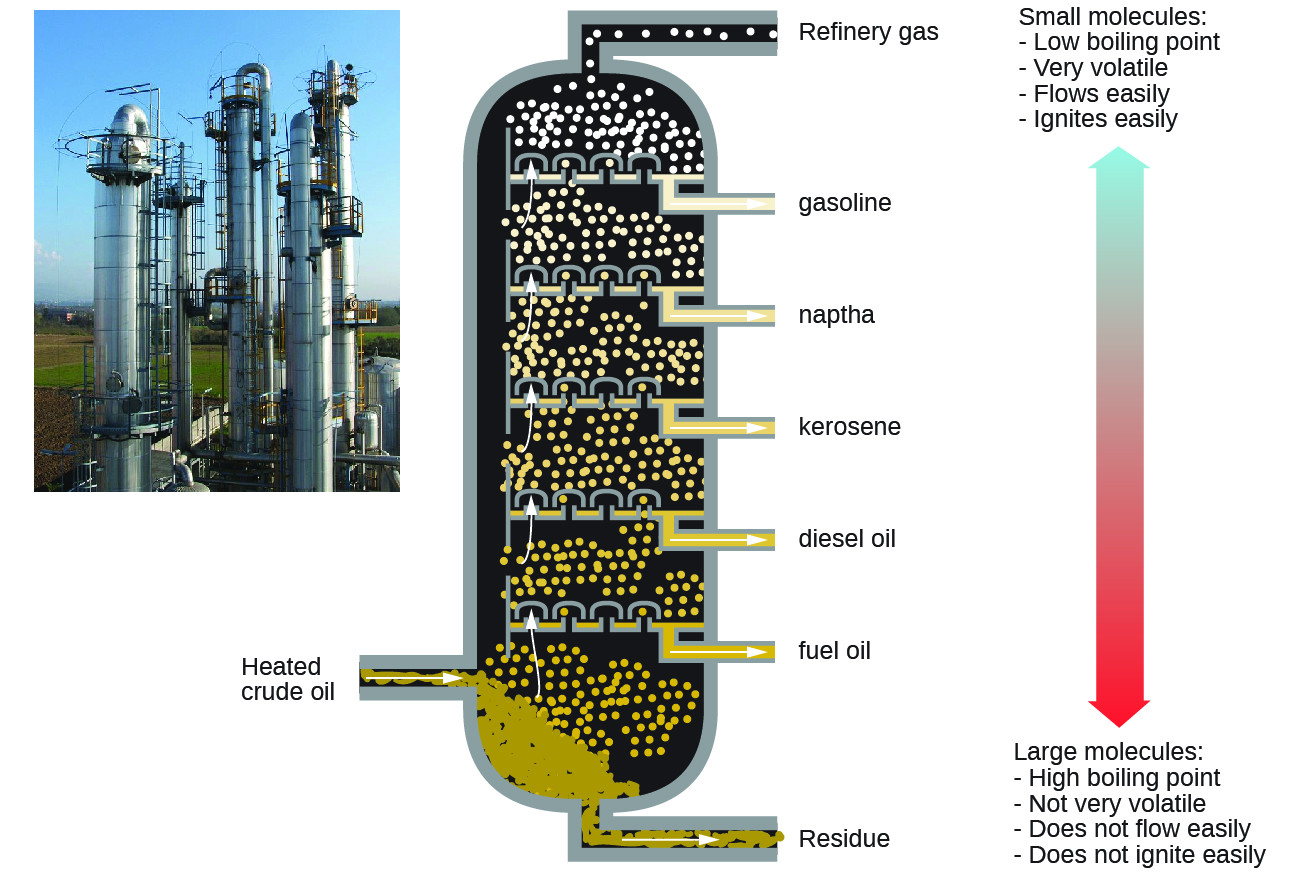
Key Concepts and Summary
The basis of all organic molecules are carbon atoms, which can form up to four strong bonds to other carbon atoms. These bonds result in chains and rings of many different sizes, shapes, and complexities that we will start to unpack in this course. The first subset of organic molecules are alkanes, which contain only single bonds between carbon atoms. In order to consistently name a molecule, it is important to always use the longest carbon chain as the root of the molecular name, giving any substituents not in the longest chain the smallest numbers possible. Common substituents tend to be alkyl groups, halogens, or functional groups.
Many molecules can form isomers, where both molecules must have the same chemical formula. The first isomer included in this unit is a structural (or constitutional) isomer which differ in their connectivity. Often, one isomer will have a longer carbon backbone chain with few substituents while the other isomer has a shorter carbon backbone chain with more substituents. Hydrocarbons are governed by dispersion forces, unless the molecule contains polar bonds from electronegative substituents.
Glossary
alkane (saturated hydrocarbon)
a hydrocarbon that contain only single covalent bonds between carbon atoms
alkyl group
a substituent that contains one less hydrogen than the corresponding alkane
cycloalkane
a cyclic alkane where carbon atoms are connected in a ring
hydrocarbon
a molecule that contain only the elements carbon and hydrogen
nomenclature
a set of rules (developed by IUPAC) to allow for systematic and consistent naming of chemical compounds
structural (or constitutional) isomer
molecules with the same molecular formula but different connectivity and spatial arrangement between atoms
substituents
branches or functional groups that replace hydrogen atoms on a carbon chain
Chemistry End of Section Exercises
- Which of the following compound can be classified as alkanes: C3H6, C6H6, C3H8, C6H12, C6H14, C3H8O
- What would the formula of an alkane containing fourteen carbons be?
- What type of intermolecular forces will be present between alkane molecules? Between cycloalkanes?
- What is the hybridization of carbon atoms found in alkanes?
- Draw structures for the following compounds:
- propane
- 3-ethyl-3-methylhexane
- 2, 2-dimethylpentane
- n-octane
- Match the following names to the structures:
i: 3-methyl-4-propyloctane iii: 3-methyl-4-isopropyloctane ii: 2, 2-difluoropentane iv: n-hexane 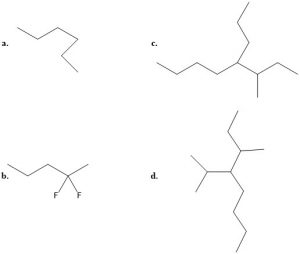
- Pentane is commonly used as a solvent in chemical reactions. Draw a bond-line structure for each isomer of pentane.
- Consider the isomers for pentane in #7, rank the expected boiling points from lowest to highest. Explain how you came up with this order.
Answers to Chemistry End of Section Exercises
- Alkanes have a general chemical formula of CnH2n+2 so C3H8 and C6H14 are alkanes.
- C14H30
- Only London dispersion forces are found between alkanes and cycloalkanes.
- sp3
- These answers are given using Bond Line Structures:
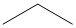
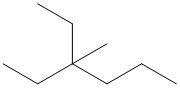
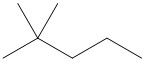
- n- indicates a straight chain alkane, with no substituents

-
- iv
- ii
- i
- iii
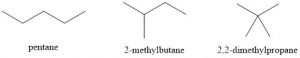
- 2,2-dimethylpropane (9.5°C) < 2-methylbutane (28°C) < pentane (36°C)
These compounds all interact with London Dispersion Forces. When comparing molecules with the same molar mass, linear molecules have more surface area and greater dispersion forces than more branched molecules. Substances with stronger intermolecular forces have higher boiling points.
