22 Enzymes
Learning Objectives
- Explain the functions of enzymes and the models that explain how substrates bind to an enzyme.
| Lock-and-Key Model | Induced Fit Model |
Enzymes
Millions of chemical reactions occur within each of our cells every minute, and many of these reactions would proceed at an extremely slow rate without catalysts. For example, the dissociation of carbonic acid that takes place in the lungs:
only proceeds at a rate of ~10-7 M/s at room temperature. However, CO2 needs to be produced in our body at a much higher rate than this. While it is theoretically possible to accelerate the reaction by raising the temperature, unfortunately, most life is only compatible with a limited temperature range.
Biological catalysts, known as enzymes, are crucial for life because they can accelerate reactions by factors of 106-1020. For example, the enzyme carbonic anhydrase accelerates the above reaction at room temperature by more than a million times.
Like all catalysts, enzymes act by combining with reactants and thereby forming lower energy transition states. In an enzyme-catalyzed reaction, the reactant(s) with which the enzyme combines is called the substrate(s). Unlike chemical catalysts, an enzyme’s interactions with substrate molecules are often partly or entirely noncovalent; that is, the interactions involve hydrogen bonding, ionic attractions, or dipole-dipole attractions.
In addition, enzyme-catalyzed reactions are highly specific to a particular substrate or a particular category of substrate molecules. Enzymes are said to “recognize” the substrate for which the enzyme serves as a catalyst, known as enzyme specificity. Enzyme interactions with substrates are sometimes so specific that an enzyme does not recognize a molecule that differs from its preferred substrate by as little as a single methyl (-CH3) group, and many enzymes will recognize one enantiomer but not its mirror image.
How do enzymes accelerate chemical reactions and achieve their specificity? The answer to both questions lies in how enzymes interact with their substrates. Typically, an enzyme is a protein molecule. Usually only part of the large enzyme molecule interacts with a substrate, and this part is called the active site.
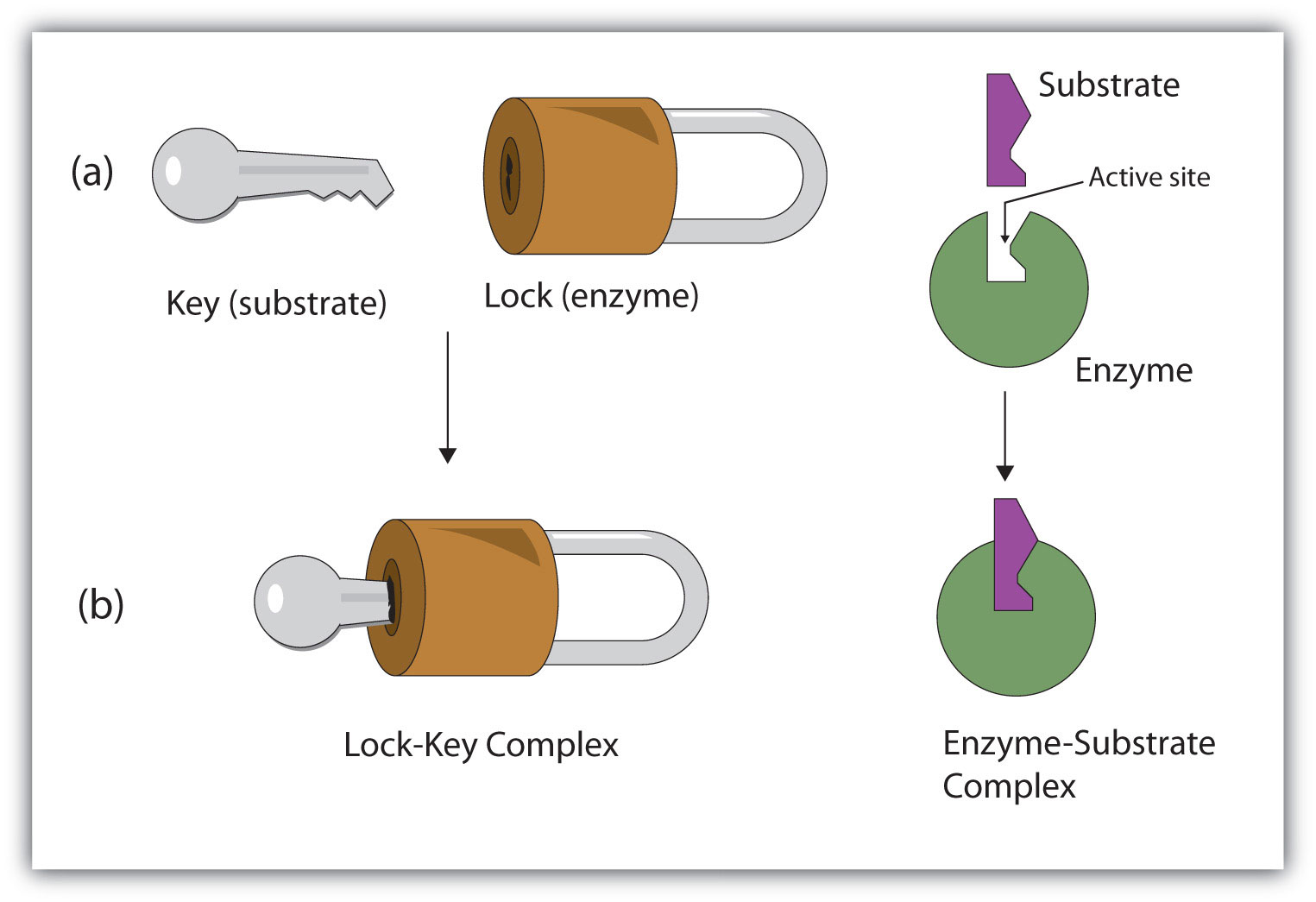
In 1890, the chemist Emil Fischer proposed that the substrate fits into the enzyme’s active site as a key fits into a lock, known as the Lock-and-Key Model. The key (substrate) has a specific molecular shape (arrangement of functional groups and other atoms) that allows it, and no other key, to fit into the lock (the enzyme’s active site) (Figure 2).
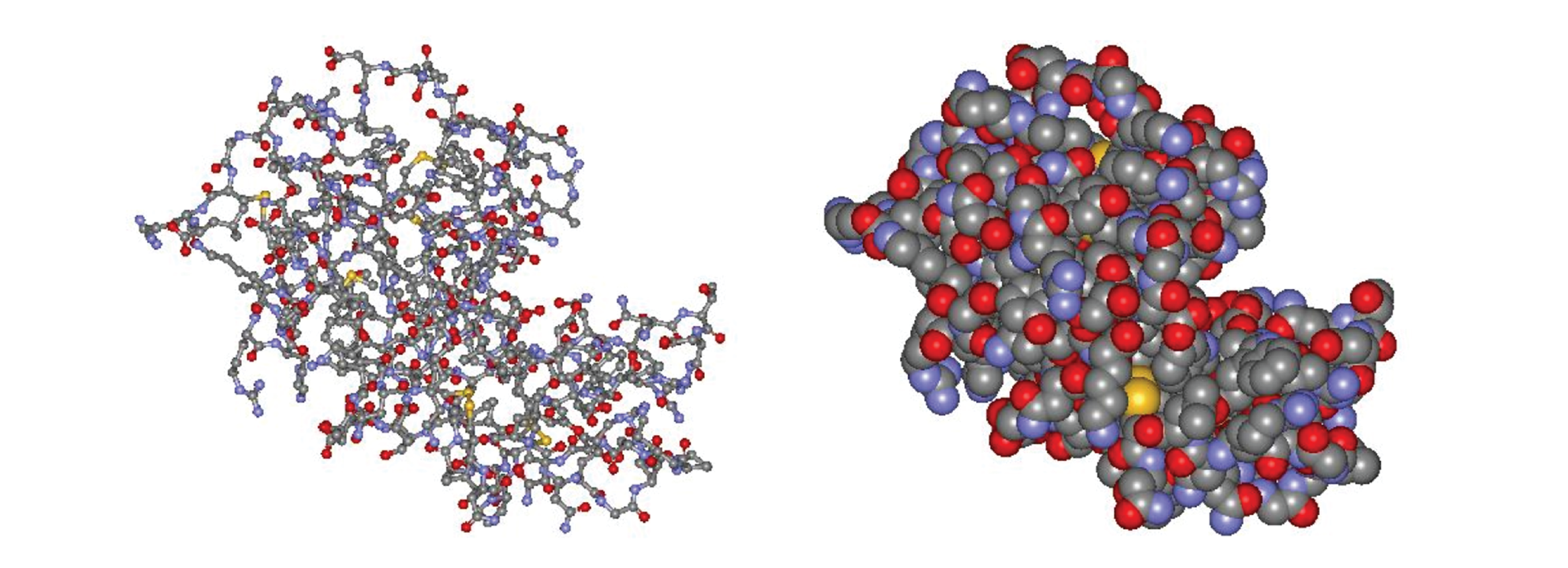
Working out the precise three-dimensional structures of numerous enzymes has enabled chemists to refine the original lock-and-key model of enzyme actions. They discovered that the binding of a substrate often leads to a large conformational change in the enzyme, as well as to changes in the structure of the substrate or substrates. The current theory, known as the induced-fit model, says that enzymes can undergo a change in conformation when they bind substrate molecules, and the active site has a shape complementary to that of the substrate only after the substrate is bound, as shown for hexokinase in Figure 3. After catalysis, the enzyme resumes its original structure.
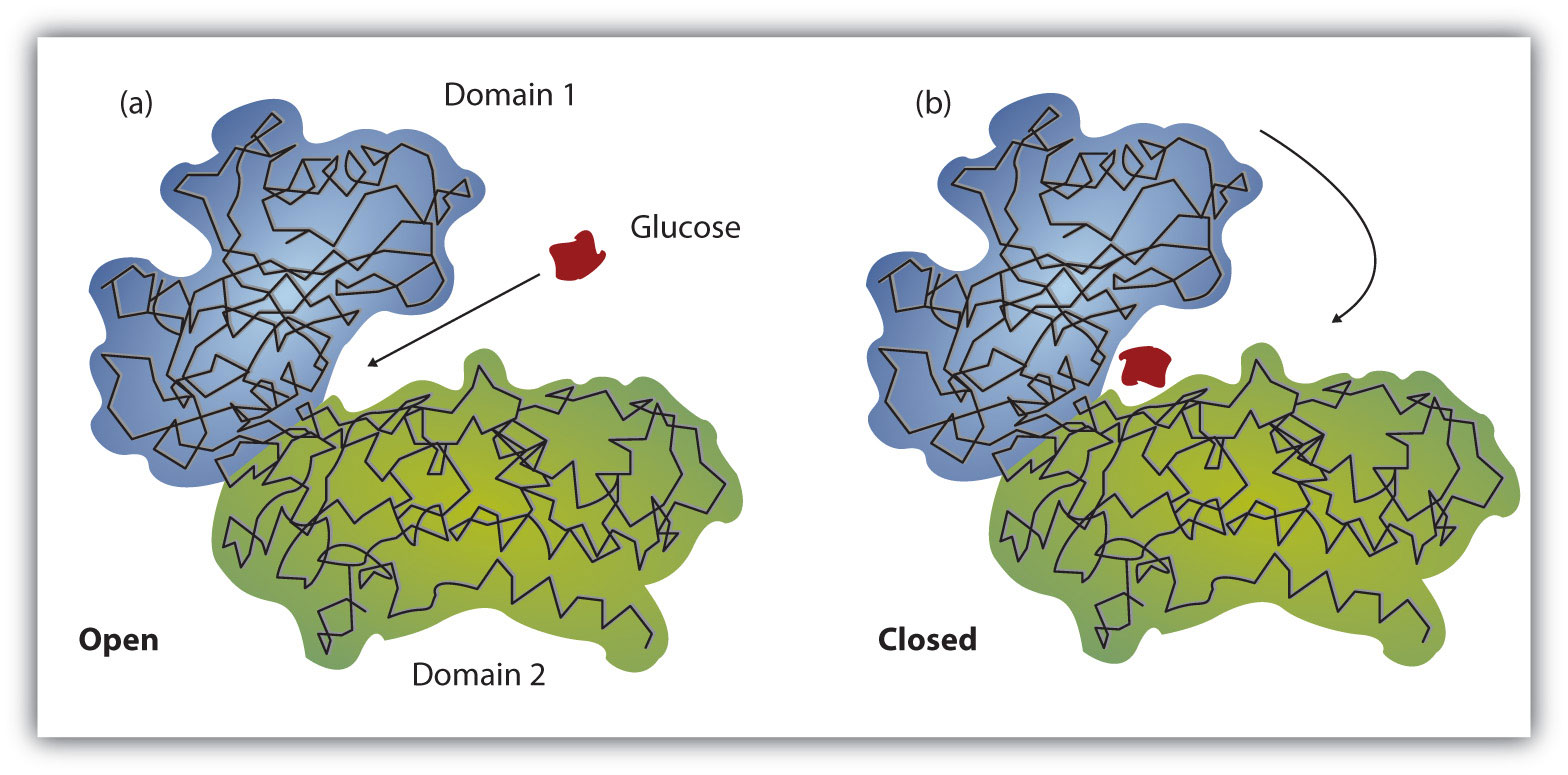
(a) The enzyme hexokinase without its substrate (glucose, shown in red) is bound to the active site. (b) The enzyme conformation changes dramatically when the substrate binds to it, resulting in additional interactions between hexokinase and glucose.
The structural changes that occur when an enzyme and a substrate join together bring specific parts of a substrate into alignment with specific parts of the enzyme’s active site. Amino acid side chains in or near the binding site can then act as acid or base catalysts, provide binding sites for the transfer of functional groups from one substrate to another or aid in the rearrangement of a substrate. The participating amino acids, which are usually widely separated in the primary sequence of the protein, are brought close together in the active site as a result of the folding and bending of the polypeptide chain or chains when the protein acquires its tertiary and quaternary structure. Binding to enzymes brings reactants close to each other and aligns them properly, which has the same effect as increasing the concentration of the reacting compounds.
Key Concepts and Summary
An enzyme is a biological catalyst, a substance that increases the rate of a chemical reaction without being changed or consumed in the reaction. A substrate binds to a specific region on an enzyme known as the active site, where the substrate can be converted to product. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. The induced-fit model says that an enzyme can undergo a conformational change when binding a substrate.
Glossary
active site
the part of a large enzyme molecule interacts with a substrate
enzyme
a biological catalyst that accelerates reactions
enzyme specificity
the “recognizability” of the substrate for which the enzyme serves as a catalyst
induced fit model
enzymes undergo a change in conformation when they bind substrate molecules, and the active site has a shape complementary to that of the substrate only after the substrate is bound
lock-and-key model
the proposal that a substrate fits into the enzyme’s active site as a key fits into a lock
substrate
the reactant(s) the enzyme combines with in an enzyme-catalyzed reaction
