13 Enantiomers
Learning Objectives
- Determine whether a molecule has enantiomers (optical isomers) and identify chiral carbon atoms.
A type of stereoisomer is a pair of molecules that are mirror images of each other but cannot be superimposed. Such stereoisomers are called enantiomers (or optical isomers), and are described as being chiral (from the Greek word cheir, χειρ, meaning “hand”). Your left and right hands exhibit chirality—they are non-superimposable mirror images of each other.
Most physical, chemical, and physiological properties of two enantiomeric substances are identical. Differences between enantiomers only become evident when the substances interact with other chiral molecules or chiral environments. (An infamous example of a physiological difference is the drug thalidomide, where one enantiomer causes birth defects but the other does not.)
Most chiral molecules have at least one atom that is bonded to four different groups, a chiral center. Chiral centers are sometimes marked with an asterisk (*) in molecular structures. A carbon atom that is a chiral center is referred to as an asymmetric carbon atom or a chiral carbon atom. For example, the carbon atom in bromochloroiodomethane is a chiral center. There are two enantiomeric isomers: the two mirror-image molecules of CHBrClI are not superimposable on top each other.
The difference between the two enantiomers may not appear to be significant, but you would have to at least partially break and re-form two σ bonds in order to go from one enantiomer to the other. Hence, enantiomeric structures represent different substances that can be separated from one another and would not readily convert from one to the other. Separating enantiomers is often difficult and requires a chiral environment: a different chiral substance that interacts differently with the left-hand molecule compared to the right-hand molecule. A macroscopic analogy is this: if all the gloves you own were mixed up, you could separate left-hand gloves from right-hand gloves by how they interact with your right hand; all gloves that fit are right-handed.
All molecules have a mirror image, but only chiral molecules are not superimposable on top of their mirror images. Contrast CHBrClI with CHCl2I (dichloroiodomethane), which is shown in the next figure. CHCl2I and its mirror image are superimposable. Thus CHCl2I is not a chiral molecule (it is said to be achiral). Two groups (the Cl atoms) bonded to the carbon atom in CHCl2I are the same, so there is no chiral center.
Usually, the easiest way to spot a chiral center is to look for four different groups bonded to an atom. The “groups” are considered in their entirety, not just the atom that is directly bonded to the chiral center. For example, a methyl group is a different group from an ethyl group, which is different from a propyl group and so forth.
Do not automatically assume that you are looking at a chiral center just because you see dashed and solid wedges in a structure. For example, lactic acid has an asymmetric (chiral) carbon atom (denoted by *) and is a chiral molecule, while isobutyric acid is not a chiral molecule.
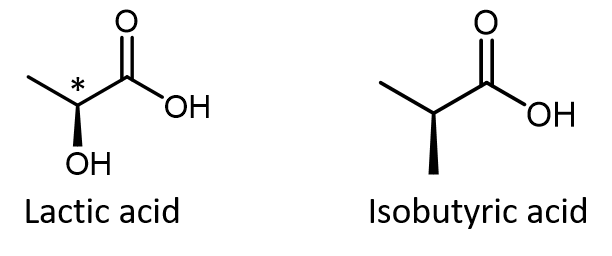
Make sure you can recognize from the wedge-dash Lewis structure that the chiral carbon atom in lactic acid has four different groups bonded to it but the corresponding carbon atom in isobutyric acid does not.
Key Concepts and Summary
Enantiomers (or optical isomers) are similar to a geometric isomer in that they have the same molecular formula and connectivity, but they are mirror images of each other. Optical isomers always have a chiral center, or a sp3 hybridized carbon with four different groups attached. It is important to look more holistically at these four groups. For example, all four of these groups could be alkyl chains which begin with carbon. The difference is the length of the alkyl group rather than the fact that each begin with a carbon atom.
Glossary
chiral carbon
a sp3 hybridized carbon with four different groups attached
enantiomer (optical isomer)
molecules that have the same molecular formula and connectivity, but are mirror images of each other caused by a chiral center and are not superimposable
