20 Protein Denaturation
Learning Objectives
- Explain what happens when proteins are denatured.
- Identify how a protein can be denatured.
As we have just discussed, the primary forces that stabilize a protein’s three-dimensional structure are:
- Sequestration of hydrophobic side chains away from water (for example, in the interior of water-soluble proteins)
- Maximizing London dispersion interactions (minimizing open spaces) in the interior of proteins
- Maximizing hydrogen bonding (for example, in α-helices or β-sheets)
- Attractions between negatively and positively charged sites formed when acidic and basic side chains lose and gain H+ ions
However, the exact process of protein folding is still a very active area of research in chemistry and biochemistry. The first hint came from the work of Christian Anfinsen on the protein ribonuclease, which breaks down RNA molecules. Anfinsen discovered that after treating ribonuclease with high concentrations of certain chemicals that cause proteins to unfold and lose their tertiary and secondary structure, the ribonuclease no longer broke down RNA. Moreover, if the chemicals were removed, the ribonuclease would spontaneously recover nearly all its RNA-hydrolyzing activity, without needing any other cellular components. Anfinsen concluded that the primary structure of a protein completely determines its three-dimensional structure at the secondary, tertiary, and quaternary levels (this is known as Anfinsen’s dogma). However, protein folding in cell is likely to be more complex than that.
The process that Anfinsen used is called denaturation, in which a protein’s native quaternary, tertiary, and/or secondary structure is changed. Proteins can be denatured by application of some external stress or compound, such as pH changes, heavy metal ions, solvent changes, radiation, or heat. All of these disrupt the multitude of noncovalent interactions present in the 3D structure of protein. Denatured proteins can exhibit a wide range of characteristics, from conformational change and loss of solubility to aggregation due to the exposure of hydrophobic groups.
| Method | Effect on Protein Structure |
|---|---|
| Heat above 50°C or ultraviolet (UV) radiation | Heat or UV radiation supplies kinetic energy to protein molecules, causing their atoms to vibrate more rapidly and disrupting relatively weak hydrogen bonding and dispersion forces. |
| Use of organic compounds, such as ethyl alcohol | These compounds are capable of engaging in intermolecular hydrogen bonding with protein molecules, disrupting intramolecular hydrogen bonding within the protein. |
| Salts of heavy metal ions, such as mercury, silver, and lead | These ions form strong bonds with the carboxylate anions of the acidic amino acids or SH groups of cysteine, disrupting ionic bonds and disulfide linkages. |
| Alkaloid reagents, such as tannic acid (used in tanning leather) | These reagents combine with positively charged amino groups in proteins to disrupt ionic bonds. |
Anyone who has fried an egg has observed denaturation. The clear egg white turns opaque as the albumin denatures and coagulates. No one has yet reversed that process. However, given the proper circumstances and enough time, a protein that has unfolded under sufficiently gentle conditions can refold and may again exhibit biological activity (Figure 1). Such evidence suggests that, at least for these proteins, the primary structure determines the secondary and tertiary structure. A given sequence of amino acids seems to adopt its particular three-dimensional arrangement naturally if conditions are right.
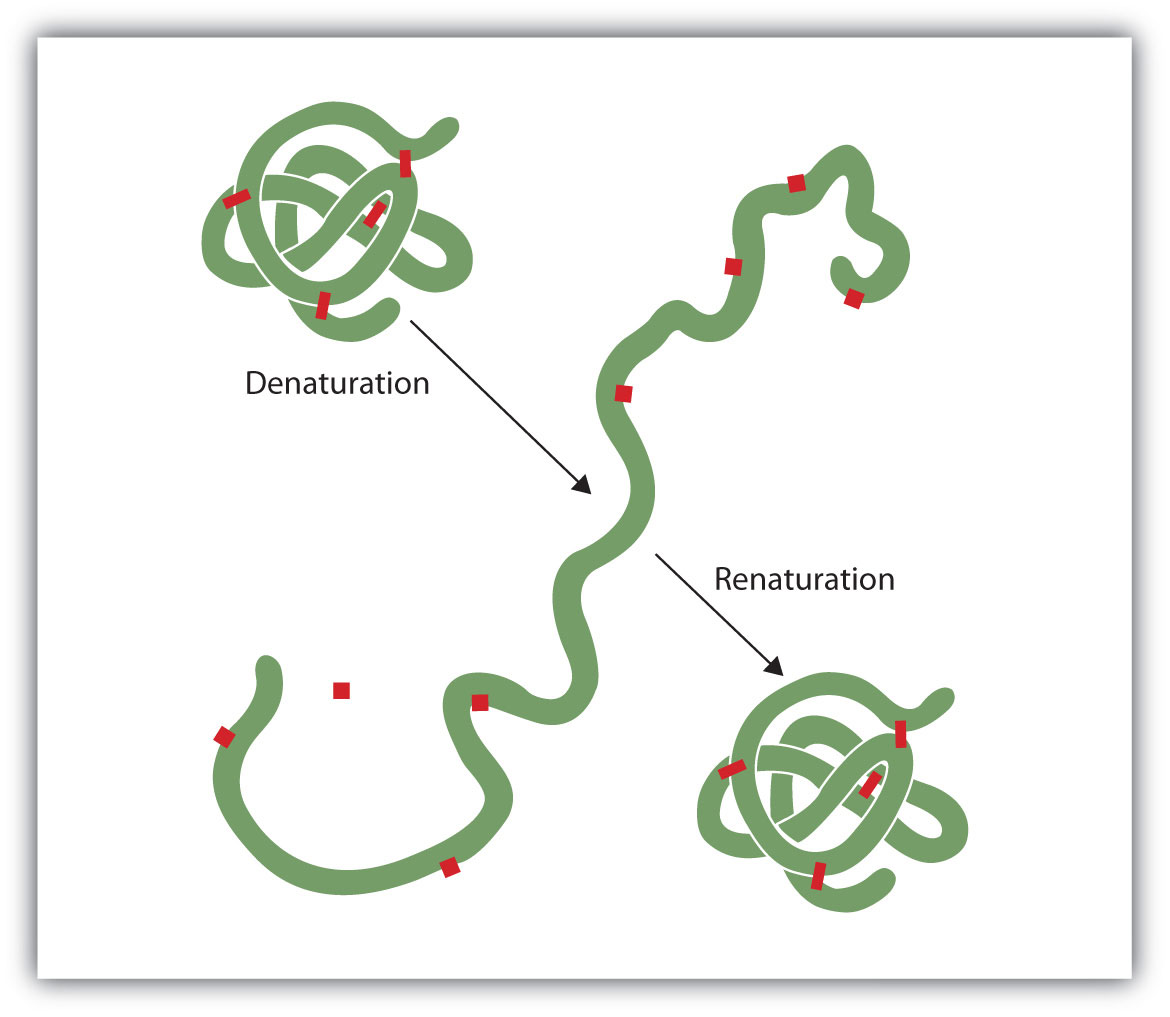
The denaturation (unfolding) and renaturation (refolding) of a protein is depicted. The red boxes represent stabilizing interactions, such as disulfide linkages, hydrogen bonding, and/or ionic bonds.
Key Concepts and Summary
A wide variety of reagents and conditions can cause a protein to unfold or denature. In general, fairly vigorous conditions are needed to hydrolyze peptide bonds. At the secondary through quaternary levels, however, proteins are quite vulnerable to attack, though they vary in their vulnerability to denaturation. The delicately folded globular proteins are much easier to denature than are the tough, fibrous proteins of hair and skin.
Glossary
denaturation
a protein’s native quaternary, tertiary, and/or secondary structure is changed
