Determination of Percent KHP in a Mixture
TOC/Help. Click to here expand/hide
Overview
Background
Pre-lab work
Experimental
Post-lab work
![]() For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
Overview
The concepts of weight percent and quality assurance are introduced in this experiment, which describes a direct titration method for analyzing an impure sample of potassium hydrogen phthalate.
Learning Objectives
- Apply a titration technique for making a chemical measurement using a colorimetric endpoint.
- Employ a control to validate a method of chemical measurement.
- Practice converting concentration units to correctly calculate the concentration of an analyte in a sample.
To cite this lab manual: “Determination of Percent KHP in a Mixture”. A Manual of Experiments for Analytical Chemistry. Department of Chemistry at UW- Madison, Summer 2024.
Visual Abstract

Background
Using Standards to Study Reactivity
There are many environmental factors that affect our everyday living. Increased CO2 abundance in our atmosphere destroys plant and marine life in oceans. Acid rain damages beautiful buildings and sculptures, eroding them away over time. Soil erosion and pollution affects groundwater and agriculture. Often times, it is easy to point to these situations as detrimental, but identifying and quantifying what is happening in order to fix the problem can be very difficult.
Chemists use a primary standard to learn accurately and precisely the concentration of solutions, such as HCl and NaOH solutions. External standards, often referred to as positive control samples, are known quantities of an analyte present in a sample used to check if a procedure or method yields the correct answer. Finding a good standard can be difficult, as you do not want it to decompose or react in unexpected ways. And the standard ideally should be present in a similar environment, or matrix, as the unknown samples. An external standard should yield the same result day in and day out, and they are especially helpful when studying a molecule that does not react predictably.
Potassium biphthalate (HKC8H4O4, KHP) is a reliable external standard used in a variety of situations, including the determination of Total Organic Carbon (TOC) content in water, which is most often used to evaluate environmental water quality. Sophisticated instrumentation, such as a mass spectrometer, can be used to quantify TOC. However, organic sources of carbon often have oxygen atoms attached. How can a scientist discern between the carbon associated with TOC verses the dissolved carbon contributed by CO2 in the atmosphere? KHP works as a great primary standard for determination of NaOH concentrations. The same reasons we choose KHP as our standardized solution for NaOH determination apply for using KHP as an external standard sample for TOC determination: namely, KHP has a large molecular weight, is non-hygroscopic, and does not absorb CO2. Therefore, part of the quality control process in understanding TOC involves checking their method for measuring TOC against a sample with a known amount of carbon, such as KHP.1
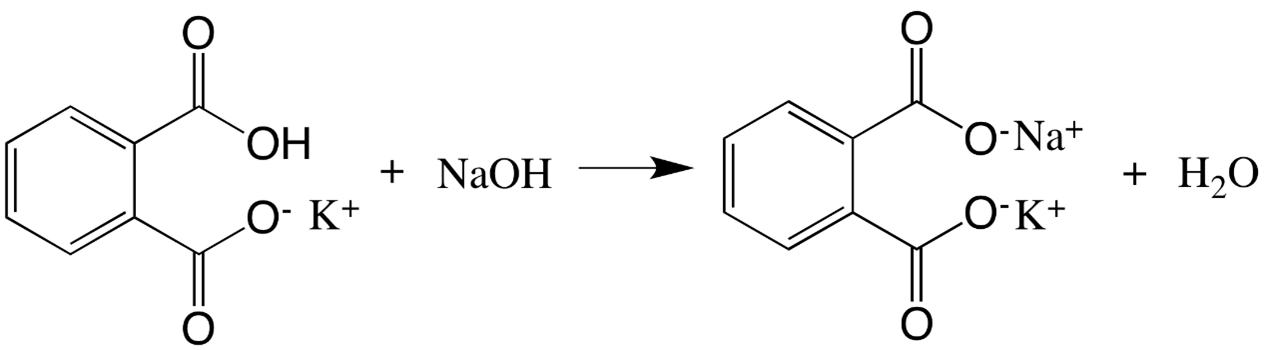
In this experiment, you will build the groundwork for applying an external standard to validate your method of quantification by studying the reactivity of KHP with NaOH, seen in Figure 1.
You will be given an unknown mixture of KHP mixed with an inert substance and, after titrating with standardized NaOH, you will calculate the % composition by mass of the KHP in the unknown. You will be graded on your accuracy to the known % composition, as well as your ability to scale your procedure to minimize waste.
In this experiment, a known NaOH solution will be used as the titrant in order to calculate the percent composition of KHP in the sample. Using the balanced chemical reaction illustrated in Figure 1, the endpoint will occur when an equal amount of moles of NaOH has been added to that of KHP. Percent composition can be calculated by taking the mass of solute (msolute) divided by the total mass (mtotal), or in this case, the mass of KHP divided by the total mass of unknown.
| [latex]\% \; composition = \left( \dfrac{m_{solute}}{m_{total}} \right) \times 100\% = \left( \dfrac{m_{KHP}}{m_{unknown}} \right) \times 100\%[/latex] | (1) |
The samples we use in this experiment were purchased from a company who produces impure samples for the purpose of teaching accurate and precise bench technique. I’m sure you would agree that careful and deliberate technique should be rewarded with high marks! In order for your results to be satisfying and effective, it is important that the samples are uniform in composition and precisely analyzed, assuring their use for the intended purposes of this experiment. Quality Assurance (QA) refers to the planned and systematic process by which a product is produced and tested for its intended purpose. Analytical chemistry is a natural fit with the concept of QA. The process used to test products must collect the right data, must analyze that data minimizing error, and should provide valuable feedback to producers on the quality, purity, and reproducibility of the production process. As you might have guessed, QA processes involve the use of an external standard, and indeed we will use an external standard in this experiment, too. The external standard in the context of this experiment is a calibration check of sorts, and is used to test the method and/or the analyst’s technique. The answer to the experiment is known in advance; the sample and matrix are equivalent to the unknown that will eventually be tested. The goal in analyzing an external standard is to prove to ourselves and, in the real world, to the users of our data, that we can achieve correct results.

Lab Concept Video
click here to hide the video (for printing purpose).
Write down your observations or notes from the video in your lab notebook.
Pre-lab Work

Lab Skills
Review these lab skills videos prior to lab.
click here to hide the video playlist (for printing purpose).
Key Takeaways
- Titrations take a lot of patience and practice to hit the end point correctly.
- By using volumetric glassware, we can improve the accuracy and precision of our measurements.

Prelaboratory Exercises
In class, you learned how to write a balanced chemical equation and use stoichiometry to convert grams of reactant to grams of products. In this lab, you will also be asked to calculate the percent composition of an unknown and the standard deviation between multiple trials.
In your lab notebook, include a detailed purpose and procedure (including the list of equipment you will need), as well as calculations to solve the following problems.
- Suppose you are using 0.1034 M NaOH to perform the procedure described in the experimental directions. Samples provided are approximately 50% KHP. Estimate the sample weight you will measure to perform the analysis. Keep in mind:
- Choose a volume of NaOH which uses a 35-45 mL range of volume in your buret.
- Allow some “wiggle room” in your volume choice. For example, if you choose 50 mL and you have more than 50% KHP in your sample, you will use more NaOH than what the buret is calibrated to deliver in one filling.
- A sample is reported to contain 44.0% by weight KHP. If you weigh 1.272 g of sample and dilute to 50.0 mL with DI water, what is the molarity of KHP in the final solution?
- Let’s say you prepared the solution in question 2, but you didn’t use a pipet to transfer DI water to the Erlenmeyer flask. Instead, you just poured from your DI wash bottle to the 50 mL line on your flask. Is this a problem? Justify your answer.

Before You Take The Quiz on Canvas
- Know the purpose of a positive control sample and understand its characteristics.
- Understand the chemistry and stoichiometry of the titration reaction.
- Be able to calculate how much KHP should be used for the titration of a sample given a set of initial parameters: approximate weight % KHP of the sample, approximate concentration of NaOH, and a target end point volume.
- Be able to calculate the precise weight % KHP of the sample given a set of titration data: mass of sample used, concentration of NaOH, and the volume required to reach the end point.

Experimental
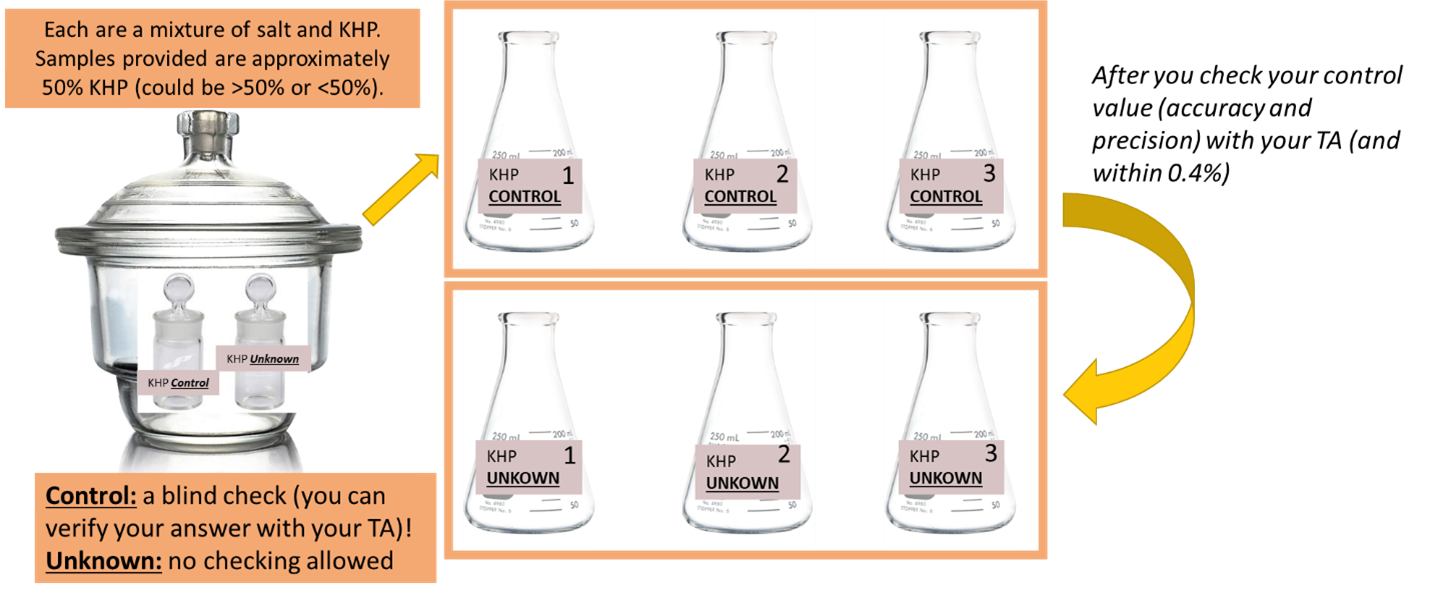
You will be given two samples to analyze. The first serves as an external standard or positive control, which is a sample used to check the accuracy and precision of your technique, reagents, instrumentation, and equipment. You should dry both of your samples and store in a prepared desiccator per the directions below. However, be sure to work with the positive control sample first.
Once you have obtained enough measurements to report your answer to your TA using an average and standard deviation, share your result with your TA, who will give you some feedback on your technique and results. For samples of about 50% by weight, the average value of a set of three titrations should be within ±0.40% KHP of the correct value. This level of accuracy would be good for an analyst in training. A master would be within ±0.10% KHP. The relative standard deviation for the positive control assay should be less than 3 × 10-3 as well. Upon confirmation of a satisfying result, continue the experiment by analyzing the unknown sample.
Preparing the Samples
- Carefully transfer all of your unknown solid into a weigh bottle. Label the container, and dry the sample at 100 -110 °C for one hour.
Be Aware: Be careful not to overheat your sample as excessive drying may cause low results due to thermal degradation.
- Weigh the estimated amount of dried sample into 250 mL Erlenmeyer flask. Be sure to accurately record the mass of your unknown with the full amount of significant figures from the balance.
- Dissolve your unknown in 50 mL distilled water that has been boiled to remove CO2. Be sure your sample is at room temperature before proceeding. (You may also air-sparge your solutions rather than boil them to take care of this procedural detail).
Initial “Range-Finding” Titration
- Fill a buret with CO2 free 0.1XXX M NaOH. This should be the NaOH you standardized in the previous experiment.
Safety Warning: If you spill NaOH on your skin, rinse off the affected area for 5 minutes under cold water while your lab partner notifies your instructor. If NaOH is spilled on the counter or floor, protect the area while notifying your instructor.
- Add 2-3 drops of phenolphthalein to your unknown solution.
- Titrate your unknown solution with NaOH until you see a pink endpoint. There is no need to be particularly accurate as this titration will allow you to scale the titration to a predictable endpoint for future trials.
- Write down the volume of NaOH used to reach the end point. This is a good spot to consider modifications to the procedure you have sketched out. If you reached the endpoint using less than 30 mL, consider weighing out more sample for the remaining titrations to better utilize the full dynamic range of the buret.
Scaled Titrations
- Weigh out the appropriate amount of your sample identified in performing the range-finding titration. Accurately record the mass of your sample with the full amount of significant figures from the balance. Dissolve the solid in 50 mL boiled, cooled (or air-sparged), DI water.
- Add 2-3 drops of phenolphthalein to your unknown solution.
- Record the initial volume of NaOH in your buret.
Be Aware: Remember to read the volumes from the bottom of the meniscus within +/- 0.01 mL and to use a stir bar or constant swirling to ensure thorough mixing during the titration.
- Rapidly titrate your sample with NaOH UNTIL YOU ARE WITHIN ABOUT 3 mL OF THE ANTICIPATED ENDPOINT. Then, decrease the rate to 1-2 drops per second and stop the titration once you observe a faint pink color. Be patient in determining the endpoint as one or two drops can have a large impact on your accuracy!
- Record the final volume of NaOH and calculate the total volume of NaOH added to your unknown.
- Repeat this process at least two more times.
Post-Lab Work Up

Results/Calculations
Fill out the answer sheet for this experiment completely. Answer the following post-lab questions.
- Is your calculated % composition (of both your control and unknown samples) accurate? Explain what sources of error lead to any inaccuracies and how they affected your measurements.
- Suppose you did not remove the bubble from the base of the buret before titrating. How would that have affected your % composition?
- Suppose there was CO2 present in your unknown solution. How would that affect your calculated % composition.

Challenge Questions
Challenge questions are designed to make you think deeper about the concepts you learned in this lab. There may be multiple answers to these questions! Any honest effort at answering the questions will be rewarded.
You and your lab mate are thinking about how to prepare your solutions for standardizing your NaOH solution. Your lab mate (A) suggests that you make a stock solution of your % KHP solution and then pipet the volume you want for each replicate, while you (B) think it would be easier to simply weigh out individual portions of KHP for each replicate. Thus, you each write your own protocol:
| Protocol A: | Protocol B: |
|
|
- Which protocol will have the least amount of error introduced? Why?
- Why does it not matter how much water we add in Protocol B (assuming the KHP dissolves)?
- Does your answer to Question 2 match your answer in Prelaboratory Question 3? Why or why not?

Lab Report Submission Details
Submit your lab report on Canvas as 1 combined PDF file. This submission should include:
- The completed answer sheet.
- Your lab notebook pages associated with this lab, which should include answers to the post-lab questions and challenge questions.
The grading rubric can be found on Canvas.
- Alberic, P. Liquid chromatography/mass spectrometry stable isotope analysis of dissolved organic carbon in stream and soil waters. Rapid Commun. Mass Spectrom. 2011, 25, 3012-3018. [link]
- Harris, D. C. & Lucy, C. A. Quantitative Chemical Analysis, 10th ed.; W. H. Freeman: New York, NY, 2020
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please ask your instructor or TA.)
