Spike Recovery and Determining a Method Detection Limit (AA)
TOC/Help. Click to here expand/hide
Overview
Background
Pre-lab work
Experimental
Post-lab work
![]() For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
Overview
This experiment explores quality assurance practices commonly used to determine the limits, accuracy, and precision of an analytical method.
Learning Objectives
- Utilize the Quality Assurance method of a Spike Recovery to reveal whether or not the sample matrix interferes with the chemical measurement of an analyte.
- Utilize the Quality Assurance method of Method Detection Limit to learn the lower limit of detection for an analysis using absorbance spectroscopy.
- Predict the suitability of a method for a particular chemical measurement based on results obtained from Quality Assurance experiments.
To cite this lab manual: “Spike Recovery and Determining a Method Detection Limit – P-Ascorbic Acid.” A Manual of Experiments for Analytical Chemistry. Department of Chemistry at UW- Madison, Summer 2024.
Visual Abstract
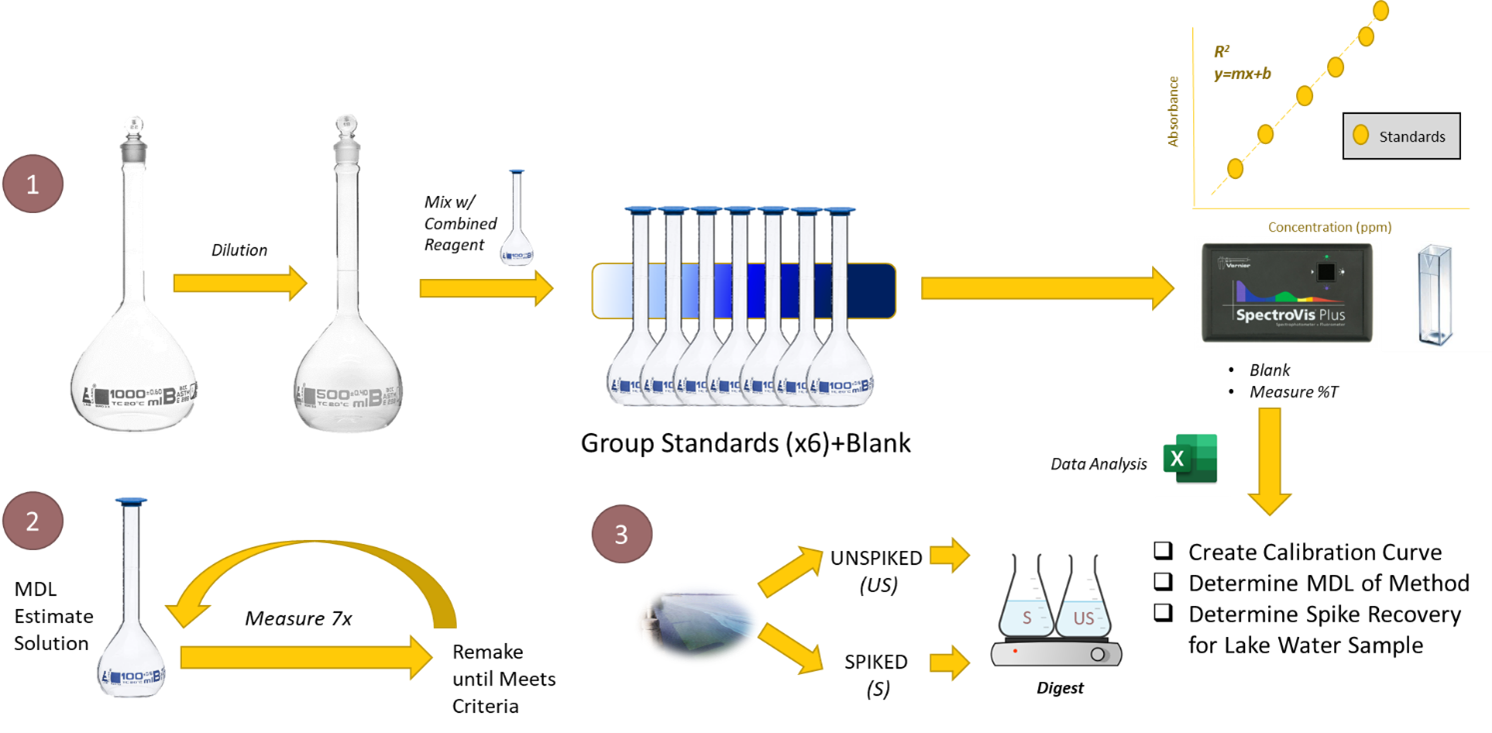

Background
The experiments you have done in this class and in your other lab courses were probably well defined, with the procedure clearly stated and the outcomes well known and explicitly defined. In fact, these experiments were once research questions which experimentalists developed to measure specific aspects of a system with an acceptable level of precision and accuracy. Quality Assurance is the practice of checking whether the right answer is achieved, essentially ensuring the user gets the right data, gets the data right, and KEEPS the data right every time a method is used.1 Method validation exists as part of quality assurance and is a means to prove a method is appropriate for all intended purposes.
Imagine you work in an environmental testing lab, and your boss has just charged you with measuring phosphorus at various sites in Lake Mendota. How do you pick the best method to do the job? How do you know whether your measurements meet a minimum level of accuracy and precision? How can you prove there are no interfering substances in your sample matrix that shifts your measured value from the true value? Quality Assurance best practices are how you would navigate these questions to ultimately defend your experimental choices and resulting data.
In this experiment, you will practice two commonly used quality assurance methods. One is the recovery of a known addition, or spike, of analyte to a sample. This method is used to determine whether a systematic shift occurs in the analytical signal of an analyte due to matrix effects. To determine the percent recovery of a spike, the sample is split into two portions and a known amount of a standard solution of analyte is added to one portion. The concentration of the analyte is determined for both the spiked, F, and un-spiked portions, I, and the percent recovery, %R, is calculated as:
| [latex]\% R = \dfrac{F - I}{A} \times 100[/latex] | (1) |
where A is the expected change in concentration due to the spike.
The second quality assurance measure is to determine the method detection limit (MDL). The MDL is the smallest measurable concentration of analyte that is statistically different from the blank. The MDL is calculated as:
| MDL = t(n-1, 99%)(s) | (2) |
where s is the standard deviation of seven or more replicate analyses, and t(n-1, 99%) is Students' t value for a 99% confidence level for a one-tailed distribution and n-1 degrees of freedom.2
Suppose you wish to measure phosphorus concentrations in the Yahara Watershed region comprising of all the lakes around Madison, to answer an experimental question. It’s important to know the limits of the Ascorbic Acid Method in measuring lake water, which is where quality assurance practices come into play. In this experiment, you will determine the MDL of the Ascorbic Acid Method for Phosphorus Determination, as well as determine whether the lake water matrix introduces any systematic error in absorbance measurements in the Ascorbic Acid Method for Phosphorus Determination.
Phosphorus occurs in lake water almost solely as phosphates, a chemical form in which each phosphorus atom is surrounded by four oxygen atoms. These are classified as orthophosphates, condensed phosphates, or organically bound phosphates. Orthophosphate, the simplest phosphate, has the chemical formula PO43-. In natural waters, orthophosphate mostly exists as dihydrogen phosphate, H2PO4¯ and hydrogen phosphate, HPO42-. Condensed phosphates are polyphosphates such as pyrophosphate, P2O74−, and metaphosphate, (PO3¯)n. Total phosphorus is the total amount of phosphorus contained, regardless of its form. To measure total phosphorus, lake samples must be oxidized (digested) to convert organic phosphates and condensed phosphates to orthophosphate.
Lake water samples will be digested using the Persulfate Digestion Method from Standard Methods for the Examination of Water and Wastewater (4500-P.B.5.). Note that in the procedure, lake water samples are digested; however, standard phosphate solutions and blanks are not. You would be correct in pointing out that if we digest the lake water sample, it would be good laboratory practice to digest the standard phosphate solutions. In the interest of time, and based on previous experience, we assume that orthophosphate is the only form of phosphorus present in standard phosphorus solutions prepared from anhydrous potassium dihydrogen phosphate (KH2PO4).

Lab Concept Video
click here to hide the video (for printing purpose).
Write down observations or notes from the video in your lab notebook.
Pre-lab Work

Lab Skills
Review these lab skills videos prior to lab.
click here to hide the video playlist (for printing purpose).
Key Takeaways
- Clean glassware will be critical to this lab, as the assay used is incredibly sensitive and can detect trace amounts of phosphorous in your glassware.
- Accurate (and precise) glassware will help you to measure the amount of iron in a sample with confidence.
- Your success using the spectrophotometer depends upon consideration of the nuances of the instrument and setting up the instrument correctly.
Extra Resources:

Prelaboratory Exercises
In your lab notebook, include a detailed purpose and procedure (including the list of equipment you will need), as well as responses and calculations to solve the following problems.
- Estimate the MDL of the Ascorbic Acid Method for Phosphorus Determination as three times the standard deviation of a method blank. Absorbance values of a method blank and the equation of the calibration line are given in Table 1. Use this value as the estimated MDL needed to prepare your MDL Standard Phosphorus Solution. Concentration units are in µg P/L.
Blank Aliquot Absorbance 1 0.007 2 0.016 3 0.018 4 0.027 5 0.009 6 0.024 7 0.013 Calibration Equation: y = 0.00064x + 0.0029 - Determine the concentrations and calculate the volumes of Standard Phosphate Solution (~ 2.5 mg P/L) from the Ascorbic Acid Method for Phosphorus Determination lab needed to prepare the standard phosphorus solutions for your calibration curve. The concentrations of standards should range from the estimated MDL to 0.4 mg P/L. These calculations should be specific to the actual concentration of Standard Phosphate Solution you made. Make a table in your notebook to reflect how the standards will be prepared.
- Calculate what the increase in phosphorus concentration will be when you add 2.00 mL of your Standard Phosphate Solution to 50.00 mL of lake water. Again, the calculation should be specific to your actual concentration of Standard Phosphate Solution. (NOTE: You do not need to consider the 2.00 mL spike in your final volume. Assume the final volume is still 50.00 mL.)

Before You Take The Quiz on Canvas
- Know what an MDL is and what information/data is needed to calculate it.
- Know what a spike recovery is and what information/data is needed to calculate it.
- Understand why it is necessary to determine the MDL and to perform a spike recovery for an analytical method.
- Be familiar with Beer’s law and how to determine concentrations using absorbance data.
- Know what matrix effects are.
- Be comfortable with the calculations and experimental process of making dilutions from stock solutions.

Experimental
Make sure to use acid washed glassware. For all glassware used, rinse with a small volume of warmed 1 M HCl (at about 40 °C) and then rinse numerous times with D.I. water. Start the acid digestion of the lake samples as soon as possible!
Spiked Lake Water Sample
Take an aliquot of at least 100 mL of lake water from the carboy in the lab. Pipette 50 mL of this sample into two 250 mL Erlenmeyer flasks. One sample will be spiked and the other will not. Lake Mendota has a phosphorus concentration of approximately 0.1 mg P/L. The spike should increase the concentration by ~0.1 mg P/L (to make the concentration ~0.2 mg P/L). Add 2.00 mL of your Standard Phosphate Solution (~2.5 mg P/L) from the Ascorbic Acid Method for Phosphorus Determination lab to one of the 50 mL lake water samples. Accurately calculate the concentration of phosphorus added in the spike to the sample.
Persulfate Digestion of Lake Water Samples
Phosphorus in lake water occurs in many forms. The ascorbic acid method only measures orthophosphate. Phosphorus in the lake samples is converted to orthophosphate using the Persulfate Digestion Method from Standards Methods for the Examination of Water and Wastewater (4500-P.B.5.).
5. Persulfate Digestion Method
- Apparatus:
- Hot plate: A 30- × 50-cm heating surface is adequate.
- Autoclave: An autoclave or pressure cooker capable of developing 98 to 137 kPa may be used instead of a hot plate.
- Glass scoop, to hold required amounts of persulfate crystals.
- Reagents:
- Phenolphthalein indicator aqueous solution.
- Sulfuric acid solution: Carefully add 300 mL conc H2SO4 to approximately 600 mL distilled water and dilute to 1 L with distilled water.
- Ammonium persulfate, (NH4)2S2O8, solid, or potassium persulfate, K2S2O8, solid.
- Sodium hydroxide, NaOH, 1N.
- Procedure: Use 50 mL or a suitable portion of thoroughly mixed sample. Add 0.05 mL (1 drop) phenolphthalein indicator solution. If a red color develops, add H2SO4 solution dropwise to just discharge the color. Then add 1 mL H2SO4 and either 0.4 g solid (NH4)2S2O8 or 0.5 g solid K2S2O8.
Boil gently on a preheated hot plate for 30 to 40 min or until a final volume of 10 mL is reached. Organophosphorus compounds such as AMP may require as much as 1.5 to 2 h for complete digestion. Cool, dilute to 30 mL with distilled water, add 0.05 mL (1 drop) phenolphthalein indicator solution, and neutralize to a faint pink color with NaOH. Alternately, heat for 30 min in an autoclave or pressure cooker at 98 to 137 kPa. Cool, add 0.05 mL (1 drop) phenolphthalein indicator solution, and neutralize to a faint pink color with NaOH. Make up to 100 mL with distilled water. In some samples, a precipitate may form at this stage, but do not filter. For any subsequent dividing of the sample, shake well. The precipitate (which is possibly a calcium phosphate) redissolves under the acid conditions of the colorimetric reactive phosphorus test. Determine phosphorus by method C, D, or E, for which a separate calibration curve has been constructed by carrying standards though the persulfate digestion procedure.
- All reagents in the Persulfate Digestion Method will be prepared for you.
- Digest your samples using a hot plate. While digesting, the samples should boil GENTLY. Monitor samples during the digestion and add distilled water as necessary to keep the solution volumes approximately the same. This practice avoids over-drying and creating noxious fumes.
- After digestion, bring the volume of the samples back up to 50 mL to match the volume of the original sample. Be careful to estimate as best as you can. We suggest marking the water line prior to digestion with tape.
- Thirty minutes of digestion is sufficient for the lake water samples.
Do not use a metal spatula when handling the ammonium persulfate. If spilt in balance, clean immediately as it is a strong oxidant and will react with metal.
Combined Reagent
To prepare 100 mL of the combined reagent, mix 50 mL 5N H2S04, 5 mL antimony potassium tartrate solution, 15 mL ammonium molybdate solution, and 30 mL ascorbic acid solution. Mix after addition of each reagent and mix in the order given. If turbidity forms in the combined reagent, shake, and let stand for a few minutes until turbidity disappears before proceeding. The combined reagent is stable for ~4 hours.
Phosphorus Standard Solutions for Calibration Curve
Prepare 6 standard phosphorus solutions ranging in concentration from the estimated MDL to 0.4 mg P/L. Explicitly plan out the concentrations of these solutions and the dilutions it will take to make these solutions in your laboratory notebook BEFORE the lab period begins. Prepare these solutions. You are encouraged to measure their absorbance while your lake water samples are digesting. You do not need to digest these standards.
Analyze Standards and Samples for Phosphorus
Combine each 50 mL of each sample with 8 mL of the combined reagent and mix thoroughly. After at least 10 minutes and no more than 30 minutes, measure the absorbance of each solution at 880 nm.
MDL Standard Phosphorus Solution
The EPA defines the MDL as the minimum concentration of a substance that can be measured and reported with 99% confidence when the analyte concentration is greater than zero. This limit is specific to a sample’s matrix and the method’s chemistry and processes. The procedure shared below is applicable to a wide variety of sample types, including a reagent blank (i.e., analyte is RO water) to wastewater containing the analyte.
- Estimate the detection limit by first evaluating the instrument noise. Basically, you need to create the data pool similar to what was presented in pre-lab question 1. Place RO water in a cuvet. Tune the instrument to the wavelength of interest. Make seven measurements of A. Convert your measured values for A into concentration units for phosphorus. Then calculate the average and standard deviation.
- The method detection limit is estimated using the information gleaned in (1) in a few different ways. You have some choices. Experience using the method or conventions preferred by the testing lab usually dictates which process to employ. Pick one:
- Multiplying the noise (this would be Ahighest- Alowest) measured in (1) by a number between 2.5-5 (whatever you wish to set the signal-to-noise ratio at) and calculate the concentration equivalent.
- Calculate the concentration equivalent of three times the standard deviation of the replicate measurements made in (1).
- Prepare 500 ml of a standard phosphorus solution with a concentration equal to the estimated MDL you determined in (2). Use your Standard Phosphate Solution (Reagent g) from the Ascorbic Acid Method for Phosphorus Determination to prepare this solution.
- Using the solution prepared in (3), create a sample to analyze using the Ascorbic Acid method. You do not need to digest these samples! Be thoughtful navigating this step. Prepare your sample for analysis. Take a few measurements and calculate the concentration of your sample all the way to concentration units. If your result lands higher or lower than the anticipated range you were targeting in (2), see the guidance in steps b and c below. To determine the MDL fully, take a minimum of seven aliquots of the sample and PROCESS EACH THROUGH THE ENTIRE ANALYTICAL METHOD. Complete all computations and corrections for any dilutions you may have performed in your estimate. This means the result you achieve is in concentration units, not units of Absorbance or %T.
- Evaluate your result. If the average result from (4) lies within the range targeted in step (2), then you have data you need to continue to step 5.
- If your result is lower than the criteria you used in step (2), use this value as your new estimate for the noise and proceed with step (2) once again.
- If your result is higher than the criteria you used in step (2), dilute your sample as needed to achieve a measurement that falls within the desired range. Perform these dilutions and collect supporting data confirming the MDL result you will report in this experiment.
- Calculate the standard deviation of the replicate measurements made in step (4).
- Compute the MDL.
Post-Lab Work Up

Results/Calculations
Fill out the answer sheet for this experiment completely. Answer the following post-lab questions.
- Using EXCEL, prepare a calibration curve using the absorbance values of the calibration standards ranging from the estimated MDL to 0.4 mg P/L. Turn in a copy of your calibration curve along with your notebook and answer sheet.
- Calculate the MDL Standard Phosphorus Solution concentrations using the calibration curve and determine the MDL.
- Use the calibration curve to determine the concentration of phosphorus in the spiked and unspiked lake water samples and calculate the percent recovery of the spike.

Challenge Questions
Challenge questions are designed to make you think deeper about the concepts you learned in this lab. There may be multiple answers to these questions! Any honest effort at answering the questions will be rewarded.
- To calculate your MDL, you can either convert your signals to concentration prior to averaging, or you can average your signals and then convert to concentration. Mathematically, these are both equivalent, but, scientifically, which one would be preferred? And why?
- After digesting the samples, you’re instructed to add water to replenish the sample volume to about 50 mL. Does the precision of the final volume matter? Why or why not?
- Suppose you carried out the above experiment and can prove the Ascorbic Acid method is an appropriate choice for measuring phosphorous to about 50 ug/L. You collect samples, digest them, make careful measurements, and work up the results. Your results reveal an answer of –15 ug/L phosphorus. Shoot! You must hand in your report and lack the time to repeat the experiment. How do you frame this answer in the reflective summary? What could have happened in the experiment to lead to your result.

Lab Report Submission Details
Submit your lab report on Canvas as 1 combined PDF file. This submission should include:
- The completed answer sheet.
- Your lab notebook pages associated with this lab, which should include answers to the post-lab questions and challenge questions.
The grading rubric can be found on Canvas.
- Nancy W. Wentworth, U.S. Environmental Protection Agency.
- Electronic Code of Federal Regulations, Title 40: Protection of Environment Part 136: Guidelines for Establishing Test Procedures for the Analysis of Pollutants, Appendix B: Definition and Procedure for the Determination of the Method Detection Limit—rev. 1.11
- Harris, D. C. & Lucy, C. A. Quantitative Chemical Analysis, 10th ed.; W. H. Freeman: New York, NY, 2020
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please ask your instructor or TA.)
