Chemical Oxygen Demand
TOC/Help. Click to here expand/hide
Overview
Background
Pre-lab work
Experimental
Post-lab work
![]() For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
Overview
Rich in color and oxidation-reduction chemistry, this lab employs a back titration technique for reporting the Chemical Oxygen Demand of a water sample.
Learning Objectives
- Apply concepts of oxidation-reduction chemistry to measure the chemical oxygen demand of a sample.
- Learn how a “back-titration” is implemented to make a chemical measurement.
- Solve a fairly complex equilibria, which employs a strong chemical oxidizer to replace O2 as the primary oxidizing agent, in order to make the chemical measurement.
- Discover how redox chemistry is responsible for the beautiful development of color and the dramatic endpoint observed at the end of the titration.
To cite this lab manual: “Chemical Oxygen Demand”. A Manual of Experiments for Analytical Chemistry. Department of Chemistry at UW- Madison, Summer 2025.
Visual Abstract
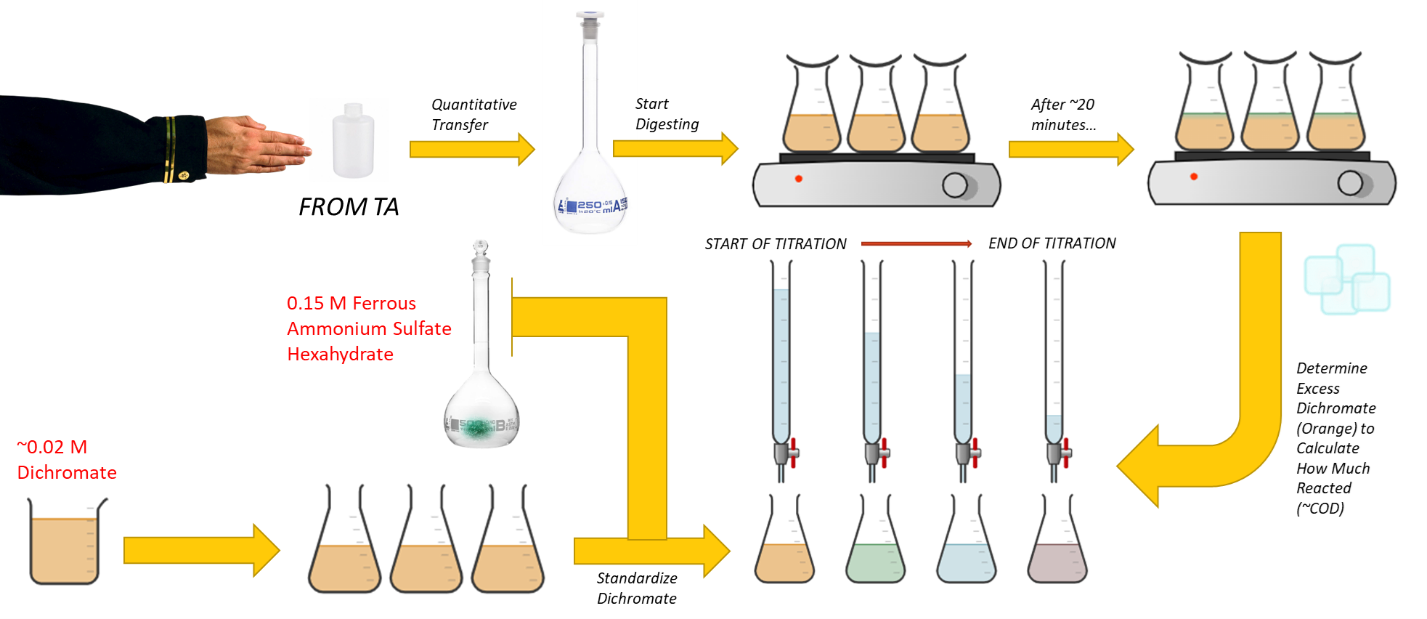

Background
The amount of oxygen dissolved in water is important to aquatic life. Decaying matter in sewage, industrial discharges, agricultural and urban runoff all use up the dissolved oxygen in water. The chemical oxygen demand (COD) is a measure of the amount of chemicals (usually organics) that consume dissolved oxygen. Biochemical oxygen demand (BOD) is a measure of the amount of oxygen consumed by the bacteria decomposing organic matter.
Water quality standards for dissolved oxygen are set by state regulations to protect aquatic life. Many lakes and streams across the country do not meet these standards. Businesses, farmers, urban developers, and non-profits can improve water quality in a variety of ways, to include changes in agricultural practices, re-vegetating stream banks, and controlling storm water to realize measurable change. For those areas affected by industrial waste water, improvements in wastewater purification are necessary.
The Redox Titration
Organic matter in aqueous samples may be determined by oxidation with dichromate. The amount of O2 that is chemically equivalent to the dichromate consumed is defined as the chemical oxygen demand (COD) of the sample. During the oxidation, in which the sample is heated with a known excess of dichromate, organic matter is converted to carbon dioxide and water while dichromate is reduced to Cr3+:
| Cr2O72- + 14 H+ + 6 e¯ → 2 Cr3+ + 7 H2O | (1) |
The excess dichromate is determined by means of an oxidation-reduction titration with ferrous ammonium sulfate using an Fe(II)-orthophenanthroline complex as an indicator. This method is called a “back titration” in analytical chemistry. The half-reaction of the iron oxidation is:
| Fe2+ ⇄ Fe3+ + e¯ | (2) |
The excess dichromate is reduced in the back titration. Subtracting the mmoles of excess dichromate in the sample from the original mmoles of dichromate added to the sample, you can calculate the mmoles of dichromate consumed by the organic material in the sample. Since it is difficult to express the concentration of the myriad of oxidizable organic substances in a sample, COD results are expressed as the amount of oxygen (in mg O2/L) necessary to carry out the oxidation of the sample to CO2 and H2O.
COD measurements are extremely useful to those concerned with water quality since they represent the amount of oxygen necessary for the aerobic biological oxidation of the organics in a water sample to CO2 and H2O if it is assumed the organics are biodegradable. The test does not, however, distinguish between oxidizable organics that are degraded and those that are not.
Any substance that reduces Cr2O72- will interfere with COD procedure. One of the most common interferences is Cl¯ which is quantitatively oxidized to Cl2 by dichromate. If chloride is known to be present in the sample, this difficulty is overcome in the method described by the EPA, where HgSO4 is added to the reaction mixture to tie up Cl¯ as a soluble Hg(II) chloride complex.
The procedure described here is applicable to samples having COD values of 50 mg/L or more. Samples are usually preserved for analysis with sulfuric acid, using about 2 mL of concentrated H2SO4 per liter of sample.

Lab Concept Video
click here to hide the video (for printing purpose).
Write down your observations or notes from the video in your lab notebook.
Pre-lab Work

Lab Skills
Review these lab skills videos prior to lab.
click here to hide the video playlist (for printing purpose).
Key Takeaways
- Accurate and precise measurements are necessary to quantify the chemical oxygen demand in this lab.
- Carry significant figures through all your calculations, and take care to round off correctly only when reporting the answer. Round off error can be an issue for this lab.
Extra Resources:

Prelaboratory Exercises
- Results of this experiment are reported in ppm O2 or mg O2/L. Thus, we must consider the reduction of O2 in determining quantities of oxygen present in our sample. Write out the oxidation reaction for H2O.
- Write out the reduction reaction for dichromate. Determine the equivalent amount of O2—in other words, derive a balanced equation that relates moles of dichromate to moles of O2. Write this equation in your notebook. For one dichromate molecule, how many electrons are consumed (or gained)?
- What is the molar ratio of the reaction between O2 and dichromate?
- Determine a balanced chemical equation for the back titration of dichromate. Write the reaction in your notebook. What is the ratio of Fe2+ to dichromate for this system?

Before You Take The Quiz on Canvas
- Understand the back-titration technique, including how and why it is used in the experiment.
- Understand the chemistry and stoichiometry of the titration reaction.
- Be able to calculate the precise concentration of potassium dichromate from a set of titration data—mass of ferrous ammonium sulfate hexahydrate and volume required to reach the end point.
- Be able to calculate the chemical oxygen demand of the sample from a set of raw data—concentration of Fe(II), concentration of dichromate, and the volume required to reach the end point.

Experimental
USE CAUTION!
- The concentrated sulfuric acid used in this experiment will burn you badly. Be attentive to adhering drops on the glassware. Remove sulfuric acid with a lot of tap water. Don’t drink it. In case of eye contact wash the eyes with flowing tap water for 15 minutes, then go to the doctor.
- Cracks in glass show up when heating; make sure you are inspecting your glassware before, during, and after for cracks.
- Ferrous ammonium sulfate should not be ingested or inhaled.
- Dichromate is extremely toxic, and very hazardous to the environment. Unused dichromate should be returned to an appropriate waste container.
- DO NOT POUR DICHROMATE DOWN THE SINK!
Begin digesting your sample as soon as you start your experiment and before performing any titrations. This portion of the lab can take up to one hour and must be done in one period. Samples must be analyzed immediately after the digestion is complete and must be done while the Fe solution is still very fresh. Further preparation for the experiment can be performed while your sample is digesting.
- Potassium Dichromate Solution. Obtain 500 mL stock potassium dichromate solution. This solution is approximately 0.02 M. Return any unused potassium dichromate solution to the stock container.
- Unknown sample. Your instructor will give you a 10 oz. plastic bottle filled with a solution containing an organic compound. Quantitatively transfer your organic containing sample to a 250 mL volumetric flask and dilute to the mark with deionized water. Mix well. Your sample has been preserved by adding 2 mL of conc. H2SO4 per liter.
- Oxidation of sample (follow the procedure below).
- Prepare at least three (four recommended) portions of your sample using the following steps:
- Pipet 50.00 mL of K2Cr2O7 into a 500 mL Erlenmeyer flask, place at least six boiling chips into the flask. Slowly add, with stirring, 50 mL of 9 M H2SO4. Note that you will standardize the K2Cr2O7 later. Cool the mixture to room temperature under a stream of tap water.
- Cautiously pipet 25.00 mL of your diluted sample into the cooled flask. Mix well.
- Using a hot plate, bring the solution to a gentle boil. Cover your sample with a small watch glass to minimize the loss of water vapor.
- Digest the sample until completely oxidized. This point is detected by observing a slight green shade, although there will still be some orange due to the excess dichromate in solution (this will take no more than an hour). Replace volume lost to evaporation with deionized water. Keep the liquid volume constant. This detail is important: If you concentrate the sample too much, sulfuric acid will decompose and create a noxious gas. Diluting beyond this liquid level will decrease the availability of protons to participate in the reaction described in equation (1).
- Prepare at least three (four recommended) portions of your sample using the following steps:
- Preparation of standard 0.15 M ferrous ammonium sulfate hexahydrate solution. Place approximately 200 mL of deionized water in a clean 400 mL beaker. Slowly add 25 mL of 9 M H2SO4 and mix. Weigh accurately the appropriate amount of ferrous ammonium sulfate hexahydrate to prepare 500 mL of 0.15 M solution. Dissolve it in the acid in the beaker, quantitatively transfer to a 500 mL volumetric flask and dilute to the mark with deionized water. Mix well.
- Standardization of potassium dichromate solution. Pipet 25.00 mL of the K2Cr2O7 into a 250 mL Erlenmeyer flask. Slowly add 50 mL of 9 M H2SO4 and mix. Allow the solution to cool and add 5 drops of ferroin indicator. The initial color of the K2Cr2O7 indicator solution is orange. Titrate with standard Fe(NH4)2(SO4)2·6H2O. The endpoint color change is quite distinct, going from blue-green to red-brown. Titrate at least two more 25 mL aliquots.
- Titration of excess dichromate in your unknown samples. Dilute the contents of the flasks to about 250 mL with deionized water. Cool the solution to room temperature and add 5 drops of ferroin indicator. Titrate the sample with 0.15 M Fe(NH4)2(SO4)2∙6H2O.
PRO TIP: Make sure you only grab the amount of reagents you actual need to do the experiment! Including:
- Sulfuric acid (H2SO4): 9M Sulfuric Acid is more concentrated and more highly corrosive than other reagents used in this course, an requires special handling for disposal. Please observe the following safety measures:
- Reduce overage. Only take the amount of sulfuric acid that you need for your experiment. You can always return to and take more. Any extra you take cannot be returned will need to be neutralized.
- Small amounts (<2 mL) left in a pipet or diluted into other solutions during the course of this lab can be disposed of down the sink. Be sure to rinse with lots of water.
- Larger amounts of excess 9M sulfuric acid should be neutralized using sodium bicarbonate. Your TA or stockroom staff can help with this process. Do not, under any circumstances, return the acid to the distribution carboy or dump it down the sink.
- If you spill any amount of 9M sulfuric acid (or of a solution containing a significant amount of it), immediately report the spill to your TA. This ensures the spill will be neutralized and cleaned up properly to prevent any injury.
- Potassium Dichromate (K2Cr2O7): Any unused (orange) solution can be recycled and should be returned to the appropriate container.
Post-Lab Work Up

Results/Calculations
Fill out the answer sheet for this experiment completely. Answer the following post-lab questions.
- Report the volume of K2Cr2O7 titrated, the volume of Fe(NH4)2(SO4) used for each titration and the final molarity calculated for the dichromate solution. Your answer should be reported in terms of an average and a standard deviation.
- Compute the number of moles of K2Cr2O7 consumed by oxidizing the organic material in each sample. Determine the number of moles of oxygen necessary to carry out the same oxidation.
- Calculate the chemical oxygen demand (COD) of your diluted sample in mg O2 per liter.
- Report the average COD value for your diluted sample as mg O2 per liter.

Challenge Questions
Challenge questions are designed to make you think deeper about the concepts you learned in this lab. There may be multiple answers to these questions. Any honest effort at answering the questions will be rewarded.
- Good news: You added the correct volume of sulfuric acid to the reaction mixture. Bad news: You grabbed the 1 M solution of H2SO4 rather than 9 M. Using the chemistry you worked out in the prelaboratory questions above as justification, predict whether the oxidation of the organic in solution will be favored. Must you start the experiment over?

Lab Report Submission Details
Submit your lab report on Canvas as 1 combined PDF file. This submission should include:
- The completed answer sheet.
- Your lab notebook pages associated with this lab, which should include answers to the post-lab questions and challenge questions.
The grading rubric can be found on Canvas.
- Methods for the Chemical Analysis of Water and Wastes, Environmental Protection Agency, Analytical Quality Control Laboratory, Cincinnati, Ohio, 1971.
- Mancy, K. H., Instrumental Analysis for Water Pollution Control, Ann Arbor Science Publishers, Inc., Ann Arbor, Michigan, 1971.
- Standard Methods for the Examination of Water and Wastewater, 12th ed., American Public Health Association, Inc., New York, N.Y., 1965, p. 510.
- Harris, D. C. & Lucy, C. A. Quantitative Chemical Analysis, 10th ed.; W. H. Freeman: New York, NY, 2020
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please ask your instructor or TA.)
