Ascorbic Acid Method for Phosphorus Determination
TOC/Help. Click to here expand/hide
Overview
Background
Pre-lab work
Experimental
Post-lab work
![]() For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
Overview
In this experiment you will determine the phosphorus (P) concentration of an aqueous sample using a standard method of analysis from Standard Methods for the Examination of Water and Wastewater.
Learning Objectives
- Interpret a technical document, extract relevant information, and develop a modified procedure for analysis.
- Perform good laboratory technique in making absorbance and transmittance measurements.
- Apply Beer’s Law to determine the concentration of an unknown chemical/compound that can be complexed with something to generate a color.
To cite this lab manual: “Ascorbic Acid Method for Phosphorus Determination”. A Manual of Experiments for Analytical Chemistry. Department of Chemistry at UW- Madison, Summer 2024.
Visual Abstract
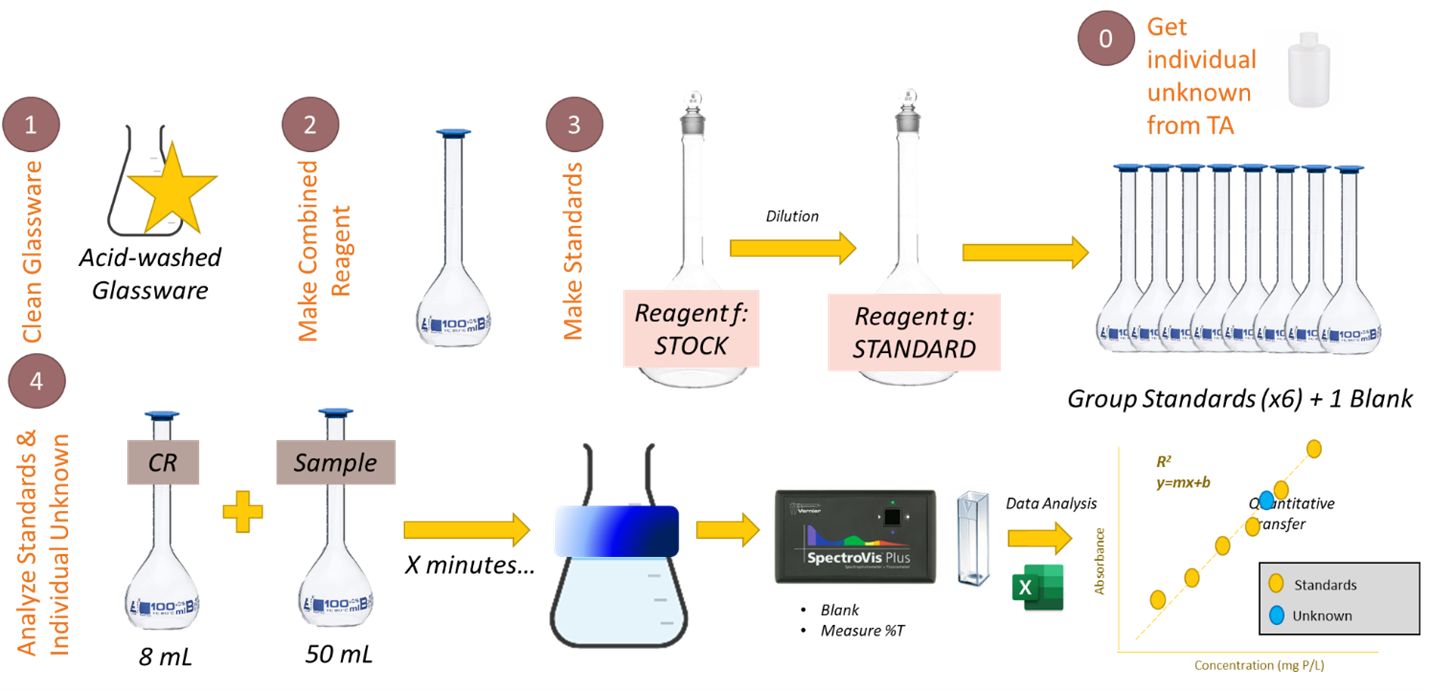

Background
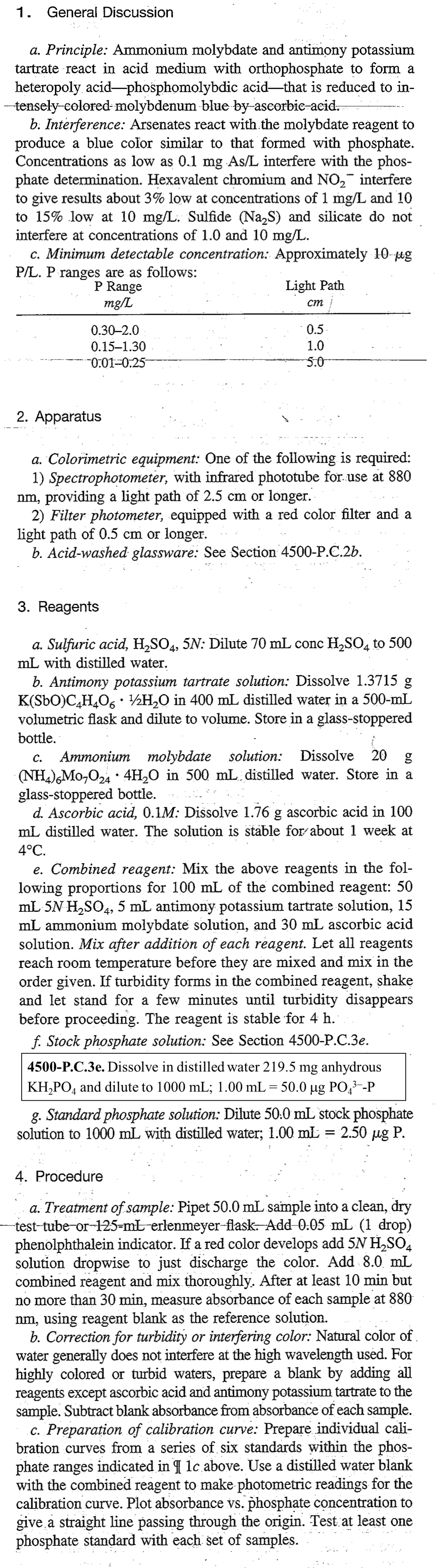
Since 1905, Standard Methods for the Examination of Water and Wastewater1 has been the standard text for water analysis. It contains hundreds of the best available, generally accepted procedures for analyzing water, wastewater, and related materials. The Standard Methods document describes three possible chemistries for measuring P: the vanadomolybdophosphoric method, the stannous chloride method, and the ascorbic acid method. The technique most commonly used is the ascorbic acid method. In this method, ammonium molybdate (NH4)6Mo7O24·4H2O) and antimony potassium tartrate (K(SbO)C4H4O6·½H2O) react in an acid medium with dilute solutions of orthophosphate to form an intensely colored antimony-phospho-molybdate complex. This complex reduces to an intensely blue-colored complex by ascorbic acid2 and the absorbance of the complex is measured at 880 nm. In this experiment, you will use the ascorbic acid method to determine the concentration of phosphorus in a water sample.

Lab Concept Video
click here to hide the video (for printing purpose).
Write down observations or notes from the video in your lab notebook.
Pre-lab Work

Lab Skills
Review these lab skills videos prior to lab.
click here to hide the video playlist (for printing purpose).
Key Takeaways
- Clean glassware will be critical to this lab, as the assay used is incredibly sensitive and can detect trace amounts of phosphorus adsorbed to your glassware. Acid washing with HCl is essential.
- Accurate (and precise) glassware will help you to measure the amount of phosphorous in a sample with confidence.
- Your success using the spectrophotometer depends upon your understanding the nuances of the instrument and setting up the instrument correctly.
Extra Resources:

Prelaboratory Exercises
In your lab notebook, include a detailed purpose and procedure (including the list of equipment you will need), as well as responses and calculations to solve the following problems.
- Your group will need to prepare a stock solution of phosphorus (P) from which you will prepare standard phosphorus solutions. How many grams of KH2PO4 (MW = 136.09) must be weighed out to make 100 mL of a 50 mg P/L stock solution? What is this concentration in mmol P/L?
- After reading the experimental procedure below, create a table in your laboratory notebook that describes how you will use the stock P solution and subsequent dilutions to prepare a set of standard solutions of P ranging in concentration from 0.1 mg/L to 2.0 mg/L. Plan to prepare a minimum of 6 standards. We recommend planning to make 50 mL each.

Before You Take The Quiz on Canvas
- Understand how to determine the concentration of an unknown solution using Beer’s Law.
- Determine the concentration of standard phosphorus solutions needed to prepare a calibration curve suitable for determining your unknown concentration.
- Know what a blank is and how to use it in spectrophotometric measurements.
- Calculate the concentration of phosphorus in a solution prepared from potassium dihydrogen phosphate (KH2PO4).
- Understand the relationship between percent transmittance and absorbance.
- Understand how a spectrophotometer works.

Experimental
The procedure in the text boxes below is copied directly from the Standard Methods for the Examination of Water and Wastewater. Working with your lab partners, you will be adapting the methods described to develop a working procedure in our lab space for measuring total phosphorus in a lake water sample. Some of the modifications and choices you’ll need to make have to do with the available equipment we have in the lab. Other choices have to do with the limitations of the experiment, which are described in the text below. The standard method text is shared on the left column of the page; guidance and considerations in your decision-making are provided on the right column of the page. You will also decide an appropriate range of concentrations for the standards. There exists no singular “right” answer to the procedure. The “right” procedure will result in a sound and reliable method for measuring phosphorus in a lake water sample.
In the end, have a clear experimental plan drafted in your lab notebook before the beginning of lab. In talking with your lab partners, you might have some differences in the details. Many times, those differences are simply a result of different choices in initial concentrations or dilution ratios; the results will work out fine. Sometimes the differences are because of a math error, or perhaps misinterpretation of the text. It’s important to talk out any differences in advance of entering the lab to minimize problems with the procedure. Be specific in how you will make solutions, including how much KH2PO4 you plan to weigh out and what the final concentrations of your solutions will be after dilution. Write your procedure in your notebook and follow it carefully while you perform your analysis.
| Standard Method Text | Manual Guidance |
 |
|
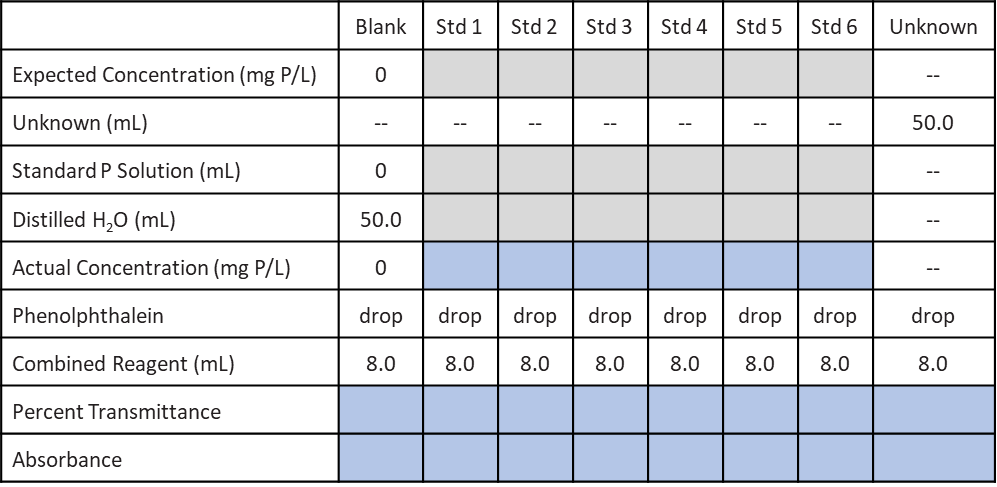
Create a table like the one below in your laboratory notebook. Fill in the rows titled “Expected Concentration”, “Standard P Solution (mL)”, and “Distilled H2O”, with the values you plan to use to make your standards. The sum of the volumes of Standard Phosphate Solution you use plus the amount of distilled H2O should add up to 50 mL. Since this table simply reflects a plan, note that you will derive the “Actual Concentration” when you record the exact mass of KH2PO4 weighed out on the balance in lab.
Are you having issues getting color to develop? Too much color or too quickly? Ask yourself:
- Did I clean my glassware correctly and enough?
- Did I confirm that I made all my solutions correctly? Did I mix them in the right order? Are the reagents I am using fresh?
- Did I wait long enough for color to develop?
You may find your group will make several shifts in the procedure as you begin preparing solutions for the experiment. In fact, this practice is a very normal event when developing a method for standard analysis. Take good notes on WHY a particular change or shift happened. For example, “The solution turned blue upon adding the combined reagent to the blank sample” might mean there was phosphorous contamination, either in the RO water you used or residual on your glassware. Your macroscopic observations are important data points in learning what does or doesn’t work. Be patient with the process! Taking good notes about your thoughts now will serve your group well later on, as you begin further modifications to the experiment for other purposes.
Post-Lab Work Up

Results/Calculations
Fill out the answer sheet for this experiment completely. Answer the following post-lab questions.
- Calculate the absorbance from the measured percent transmittance values and determine an average absorbance value for each of the solutions measured.
- Use EXCEL to prepare a calibration curve of average absorbance versus phosphorus concentration. Make sure to include the point from the blank you prepared. Turn in a copy of your calibration curve along with your notebook pages and answer sheet.
- Using the calibration curve, determine the concentration of phosphorus in your unknown solution. Report the concentration of the unknown in units of mg P/L and mmol P/L.

Challenge Questions
Challenge questions are designed to make you think deeper about the concepts you learned in this lab. There may be multiple answers to these questions! Any honest effort at answering the questions will be rewarded.
- Lake water samples can sometimes contain particulate matter, sludge, algae, and other complexed chemicals that result in colored or cloudy (turbid) samples. Describe two possible modifications to the methods above to accommodate samples that are colored or turbid.

Lab Report Submission Details
Submit your lab report on Canvas as 1 combined PDF file. This submission should include:
- The completed answer sheet.
- Your lab notebook pages associated with this lab, which should include answers to the post-lab questions and challenge questions.
The grading rubric can be found on Canvas.
- Eaton, A.D.; Clesceri L.S.; Rice E.W.; Greenberg A.E.; Franson M.H., Eds., Standard Methods for the Examination of Water and Wastewater: 21st ed.; American Public Health Association: Washington, DC, Water Environment Federation: Alexandria, VA, and American Water Works Association: Denver, CO, 2005.
- Murphy, J.; Riley, J.R. A modified single solution method for the determination of phosphate in natural waters. Anal. Chem. 1977, 27, 31-36.
- Harris, D. C. & Lucy, C. A. Quantitative Chemical Analysis, 10th ed.; W. H. Freeman: New York, NY, 2020
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please ask your instructor or TA.)
