Good Laboratory Practice
TOC/Help. Click here to expand/hide
Overview
Background
Pre-lab work
Experimental
Post-lab work
![]() For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
Overview
This first experiment serves to orient you to the “essential tools” for measuring volumes and masses, preparing solutions, and keeping the laboratory equipment in top performance condition.
Learning Objectives
- Clean and store glassware and hardware safely and properly.
- Choose the correct vessels or glassware for transferring liquids.
- Gain practice working with weighing and using the balance.
- Perform safe chemical handling in lab when either preparing chemicals or making manipulations.
- Use glassware as intended for either making a total volume of solution or transferring an accurately measured volume from one vessel to another.
To cite this lab manual: “Good Laboratory Practices”. A Manual of Experiments for Analytical Chemistry. Department of Chemistry at UW- Madison, Summer 2024.
Visual Abstract

Background
The practice of chemical measurement is a nuanced science. Chemical measurement is a skill developed over time by learning and attending to all the details important to a successful experiment. Mastery involves not only theoretical comprehension, but also the development of dexterity in transferring solids and liquids, and the ability to select and use the appropriate glassware or equipment for each specific task.
The collective term for describing the acceptable practice for chemical manipulations in the lab is “Good Laboratory Practice” (GLP), and broadly covers the following topics:
- How to clean and store glassware and hardware;
- Choosing the correct vessels or glassware for transferring liquids;
- Adding reagents in a systematic way to perpetuate the desired reaction safely and efficiently with minimum contamination and error;
- Safe chemical handling in lab when either preparing chemicals or making manipulations;
- Using glassware as intended for either making a total volume of solution or transferring an accurately measured volume from one vessel to another.
This experiment serves as your orientation to common “GLPs” using some of the more common equipment and glassware in the lab. You will build on these foundational topics throughout the semester and will continue to learn important nuances to these techniques for specific manipulations as you navigate the lab experience for the semester. Read the information below carefully and be ready to demonstrate to your TA the skills described in the Experimental section of this manual.
1. Housekeeping
Left-click here to expand HOUSEKEEPING section.
Good housekeeping is important for everyone’s safety, including our janitorial staff, who are not chemists. The analytical lab is a shared space and a busy place. Any mess you don’t clean up will surely inconvenience more than a few people and could, in fact, hurt someone else. Leaving chemical spills for others to tend to is not only unsafe, but also provides a sure source of contamination to experiments, clothing, and skin, as well as unintended reactions should another reactive chemical spill on top of it. Messy benches won’t impress future employers looking for careful, detail-oriented, and safety-minded staff members to run a lab space. If you spill something, clean it up.
Put chemicals and equipment away immediately after you use them. Use a paper towel to wipe off the outside of reagent bottles before returning it to the station. If you approach a station to make a measurement, such as a balance station, inspect the space. Clean it if you notice any spill or spots. When you’re finished using the space, leave it in as good or BETTER condition than when you found it. Fume hood stations are premium real estate in our lab space. Once you’re finished with whatever measurement or chemistry you needed the hood for, remove your materials and use a damp paper towel to wipe down the bench.
Sometimes we will use hot plates to heat a reaction. Pay careful attention to whether you should be simply warming a solution, or if the reaction must come to a full boil. If a full boil is required, it is often helpful to add a few boiling chips to the solution to avoid the flasks or beakers from bumping on the plate. When first heating the solution, choose a medium temperature to start. If you choose a temperature that is too high, you risk burning the solution and ruining the experiment. Choosing too low of a temperature may result in a limited or absent reaction. Be observant about the boiling process and tune the temperature frequently to optimize the result. You must monitor the reactions you’re heating 100% of the time. Stay at the bench. Watch the reaction (avoid doing homework or reading a book!). Log any macroscopic observations in your lab notebook, such as color change, gas evolution, fizzing, bumping, or splashing. These observations may provide context for a result you obtain later on. When removing flasks or beakers from the plate, use tongs or an oven mitt to protect your skin from the hot glass. Avoid placing your whole hand over the beaker or flask, as the steam from the solution is hot and can burn your skin.
When using chemicals and supplies, use all of one container before opening or acquiring a new container, unless you’re explicitly directed to do otherwise by your TA. Place empty boxes in the trash. If you have an excess amount of chemical and there are no explicit instructions on how to dispose of it, ask your TA or look up the Material Safety Data Sheet (MSDS) for the reagent to learn if it can be washed down the sink. NEVER return unused reagent to the bottle you took the reagent from.
At the end of the laboratory period, leave clean glassware in your drawers for next time. Dispose of any remaining trash, and use a paper towel to wipe your bench space before leaving the lab.
Upon leaving the lab, wash your hands.
2. Water in the Lab
Left-click here to expand WATER IN THE LAB section.
The lab area has two types of water available for your use.
- Tap water: for washing glassware, your hands, or wiping your bench space.
- Deionized water: anytime you’re making a solution that you plan to use to perform chemistry.
- Identified in the lab by the swan-necked spout and a white faucet cap labeled “DI”.
- Madison tap water is notoriously “hard”, meaning it contains a high mineral content including calcium and iron. While hard water is fine for drinking and (with the right soap) fine for washing things, hard water is not a good option as a solvent for chemical reactions.
3. Identifying and Caring for Glassware
Left-click here to expand IDENTIFYING AND CARING FOR GLASSWARE section. (includes Table 1)
Glassware is generally characterized as either volumetric or non-volumetric.
- Volumetric glassware is calibrated to measure a defined volume or a range of volumes accurately and precisely. The quality of this glass is soft, since the glass must usually be blown professionally to achieve the desired volume for its purpose.
- Non-volumetric glassware, on the other hand, is used to store solutions or chemicals as well as (but not limited to) mixing, heating, and performing chemical reactions. Non-volumetric glassware is hard and hardy to withstand the rigors of chemistry.
Because of the different qualities of the glass, volumetric and non-volumetric glassware use different soaps for cleaning purposes. When cleaning your volumetric glassware, use microcleaner soap, which is a low-phosphate, low acidic soap that is more friendly for cleaning the soft glass. To use this soap, draw up a few milliliters of warmed soap solution into your flask or pipet. Agitate the solution. Drain and repeat. Rinse the inside of the flask or pipet using 3-5 rinses of tap water. Let your final rinse be with DI water.
Non-volumetric glassware, and any other equipment in your drawer needing a wash for that matter, can be cleaned using the powder soap in the lab. Usually the bottle says “Comptrex” or “Alconox”. To use this soap, add about ¼ cup of the soap to a wash bin. Fill the bin with warm water to create a soap solution. Then go about washing the glassware and equipment much like you would wash your dishes at home. It’s fine to wipe the inside of the beakers. When finished, rinse with tap water 3-5 times. Let your final rinse be with DI water.
All glassware should be dried on the OUTSIDE only using a cheese cloth. You’ll be given one cheese cloth for this purpose at the beginning of the semester. Use and reuse this cloth for this purpose only. Don’t use it to wipe your bench area, for example. Replacement cheese cloths will generally NOT be available, so take care of yours.
Volumetric glassware, such as pipets and burets, are designed to “deliver” an accurate and specific volume of solution. These pieces of volumetric glassware are usually labeled with “TD” on the neck of the glassware, to identify that the glassware is meant to deliver that volume, and therefore is calibrated to retain a small amount of the solution in the tip of the pipet or buret. Volumetric flasks are designed to “contain” an accurately defined volume (marked on the neck as “TC”), meaning that when the flask is filled with the meniscus properly aligned to a mark, the volume of the solution contained in the flask is accurately known.
Volumetric pieces of glassware promise volume accuracy to a standard number of significant figures (4), and the first uncertain digit in the volume measurement is labeled as the tolerance for that piece of glassware. These volumes and tolerances are set and guaranteed by the manufacturer of the glassware, although the volumes and tolerances tend to be standard across the industry. Volumetric glassware that does not meet ALL the quality assurance parameters of the manufacturer is often still sold by the manufacturer, but identified as “Class B” rather than “Class A” glassware. In our experience, most Class B glassware meets the Class A volume and tolerance criteria, although perhaps the volume line is low or high on the neck compared to Class A glassware. These volumes and tolerances can be verified by a calibration technique, which is shared in the “Experimental” section. Table 1 lists the information for glassware made by Kimball-Kontes.
| Pipet | Volumetric Flask | Buret | |||
| Capacity | Tolerance | Capacity | Tolerance | Capacity | Tolerance |
| 1 | 0.006 | 1 | 0.010 | 5 | 0.01 |
| 2 | .006 | 2 | .015 | 10 | .02 |
| 3 | .01 | 3 | .015 | 25 | .03 |
| 4 | .01 | 4 | .020 | 50 | .05 |
| 5 | .01 | 5 | .02 | 100 | .10 |
| 10 | .02 | 10 | .02 | ||
| 15 | .03 | 25 | .03 | ||
| 20 | .03 | 50 | .05 | ||
| 25 | .03 | 100 | .08 | ||
| 50 | .05 | 200 | .10 | ||
| 100 | .08 | 250 | .12 | ||
| 200 | .10 | 500 | .15 | ||
| 100 | .30 | ||||
| 2000 | .50 | ||||
| Tolerances confirmed - Quoted by Kimble Kontes Technical Document, Class A & Class B Tolerances | |||||
The volume occupied by a given mass of liquid varies with temperature, as does the volume of the container holding the liquid. As a general rule, volumetric glassware should not be heated because the calibration can be permanently altered.
Before using any piece of glassware, whether it’s volumetric or non-volumetric, you should always inspect the glassware for any chips or cracks that may either cut you or perhaps compromise the safety of the glassware itself when exposed to liquid, chemicals, or heating. If you find a piece of glassware broken in your drawer, ask your TA or the stockroom staff for a replacement piece.
You can identify clean glassware by noting that the water “sheets clean” on the internal surface of the glass. Spots or droplets (one or two drops at the neck is okay, but more is not) that cling to the glass indicate the glassware is dirty or etched from previous contents. Once you trust you have a clean set of glassware, the maintenance for maintaining that quality is minimal. After you have made the necessary measurements, rinse your glassware (both volumetric and non-volumetric) with 3-5 small aliquots of cold water. Let your last rinse be with DI Water. Wipe the outside of your glassware dry with the cheese cloth, and leave the rest to drip dry in the drawer. Never let a reagent dry onto the side of a volumetric piece of glassware, as it can etch the glass. Volumetric flasks can be capped off with a little DI water, to keep the internal surface wet and ready to accept future solutions with minimal preparation.
4. Dessicators and Drying Ovens
Left-click here to expand DESSICATORS AND DRYING section.
Most solids tend to absorb at least some atmospheric moisture and, as a consequence, small systematic errors in the mass measurement of a solid can accumulate to foil your experiment. This effect can be substantial when a large surface area, such as a fine-powdered reagent, is exposed to a humid atmosphere. To eliminate weighing errors, solids are often dried in an oven to drive off excess water molecules, then stored in a desiccator to prevent further exposure to atmospheric moisture. Some samples can degrade if overheated or heated over too-long a duration. Prudent practice is to check a resource about the reagent you’re interested in (such as the Merck Index), to learn the best method (temperature and duration) for drying the reagent of interest.
Before drying a chemical, you need to have a prepared desiccator to put it in. Desiccators serve as containers to store chemicals with some protection from the lab atmosphere. They can be made from a variety of material—metal, plastic, glass—and use vacuum grease as a way to provide a moisture barrier between the atmosphere and the internal area of the desiccator. Internal to the desiccator are beads of desiccant, comprised of hydrophilic material to quickly absorb any moisture internal to the desiccator.
You will have your own personal desiccator to use for the semester. During the first lab period. you should transfer the desiccant to a 500 mL beaker. Note how the pieces of the desiccator assemble as you’re pulling it apart by drawing a picture in your laboratory notebook. Dry the desiccant in the 160-degree Celsius oven for about an hour. While the desiccant is drying, you can clean your desiccator with the powdered lab soap solution, but be sure not to submerge your desiccator or its lid completely. Be careful not to wash or rub away the vacuum grease that creates the seal. Dry the desiccator thoroughly. Place the dried desiccant in the bottom of the desiccator, and then reassemble the other pieces to resemble the apparatus as you found it. If you are concerned about the seal, you can add a small pea-sized amount of grease.
To dry a chemical in a drying oven, use a clean and dry weigh bottle. Transfer the solid you plan to dry to the weigh bottle. Prepare a clean and dry 250 mL beaker with a label, that lists your name, date, and the reagent name. Place the weigh bottle AND THE CAP in the beaker.
Do not put the cap ON the weigh bottle while in the oven! If you do this, the water you drive off with heat will patiently wait right above the surface of the chemical you intend to dry, only to re-adsorb.
And remember the ideal gas law? What happens to the atmosphere when heated? If you guessed that air expands, you are correct. If the cap is on while heating, the gas will expand and escape. Then when you remove the reagent from the oven to cool, the air will contract as it cools and pull the cap down with it, sealing your container and making it very difficult to ever open the weigh bottle again. So, keep the cap off!
Place the beaker with your reagent in the oven on either the top or second from the top shelf. Avoid using the bottom, as the heating element is there and sometimes can overheat your sample. As you place your material, take care to avoid touching the interior surface of the oven as it is very hot and can burn your skin. Keep the oven door closed for the duration of the time you need to dry the chemical. When removing your beaker from the oven, use tongs or an oven mitt. Bring the beaker to your station and transfer the weigh bottle to your prepared desiccator. Keep the cap off the weigh bottle until the entire apparatus has reached room temperature.
5. Measuring Liquids
Left-click here to expand MEASURING LIQUIDS section.
Accurate measurements of volume are performed by using glassware such as a pipet, buret, or volumetric flask. Volume can also be determined by weighing the mass of the liquid and converting to volume using the density of the solvent, which you will practice later when you calibrate a few pieces of glassware. Volumetric equipment is marked by the manufacturer to indicate the manner (e.g., “TD” versus “TC”) and the quality (e.g., Class A or B) of the calibration. A general description of technique for using volumetric glassware is described for some of our most common pieces below.
5.1 Preparing for Volume Measurements Using a Pipet or Buret
A good volume measurement starts with good preparation! Gather the necessary glassware, which should include a beaker to contain the solution you wish to measure and a larger beaker to hold your waste. Appropriately choose the size so that you can easily draw up the desired volume. Have paper towel and Kimwipes on hand to catch any spills. Have a wash bottle nearby. Add a few milliliters of the solution you wish to measure to the receiving beaker. Swirl the solution and dispense to the waste beaker. Do this three times, before filling the receiving beaker with slightly more than the volume you need. For pipetting or filling a buret, plan to have sufficient excess to ensure that no air is taken up into the pipet. Also have a bulb on hand for drawing solution into a pipet and/or a funnel for pouring from the beaker to the mouth of the buret.
5.2 Preparing for Volume Measurements Using a Volumetric Flask
Preparation for this technique includes having a clean beaker of sufficient size filled with your solvent. Also have on hand a funnel to transfer the liquid, the desired volumetric flask, and a wash bottle and/or eye dropper (also known as a “plastic squeezy baby”) for filling the flask to the meniscus line. Ideally, a volumetric flask should be wet before using. If you’re using a dry flask, rinse it three times with DI water before beginning your measurement. If you’re making a solution by dissolving a solid, be sure you have accurately weighed the reagent you plan to use, logged the mass in your lab notebook, and it’s near your station.
5.3 Pipets
Most of the work in this course will involve the use of volumetric pipets for measuring a fixed volume of solution to transfer to other solutions or containers. Pipets that show gradients of volume along the axis to the tip, called Mohr pipets, can deliver a range of amounts, but the quality of the measurement becomes very dependent on the hands of the user. Take care you are accurately recording the actual volumes dispensed by Mohr pipets into your lab notebook.
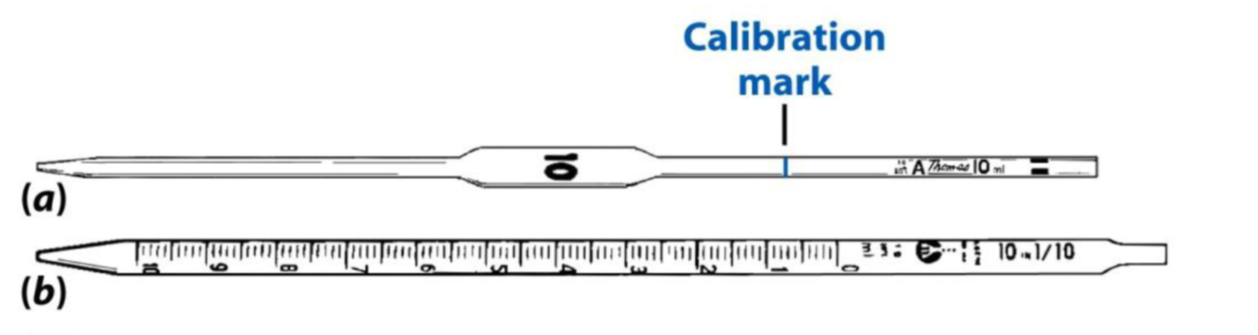
You should always use a bulb to draw up solution into the pipet. The bulb fits opposite the tip-end of the pipet. In using either pieces of glassware, you should wet the interior of the pipet several times with your solution. Do so by drawing up a mL or two of solution into the pipet, and then carefully rolling the solution around the interior to fully wet the surface. Do this three times.
For the final fill, draw up a good inch past the calibration line on the neck, and using your fore finger, create a seal over the hole to retain the liquid. By moving your finger gently forward and back over the hole, you will create small pockets of air, which gives you fine control in adjusting the meniscus to the calibration line. This part is tricky and takes patience! The fore finger truly does work better than using your thumb, as your thumb has more tissue that fully fills the hole. If the meniscus falls below the line, replace your finger with the bulb and refill the pipet.
Once you are satisfied with the location of the calibration line, move the pipet to the receiving vessel and lift your finger. Let the solution drain into the receiving flask. Be sure to touch off any remaining drop that lingers on the pipet tip to the side of the receiving flask. You can also rinse the drop off with a little squirt from your wash bottle. Be sure to rinse the pipet with DI water before putting it away.
5.4 Volumetric Flasks
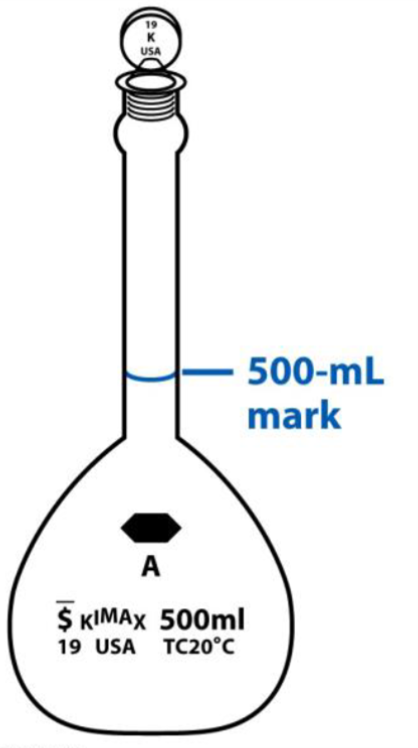
Add about 10 mL of solvent to the volumetric flask, using a funnel to transfer the liquid through the narrow neck. If you’re adding solid reagent, add that next, using a wash bottle of your solvent and a funnel to complete the quantitative transfer of reagent to the flask. Cap the flask and invert several times, noting whether the reagent has dissolved or not. If you plan to add an aliquot of liquid, use a pipet for the most quantitative measurement of the desired volume. (Take care not to force the bulb through the narrow neck of the flask, and also take care not to drop the pipet completely into the flask! Be sure to simply touch off the drop at the end of the pipet).
Fill the flask to about 2/3 full, firmly replace the cap and invert the flask several times again, noting whether there continues to be undissolved reagent, or perhaps precipitated reagent developing in the flask. If there is unwanted solid in the flask, you can try to use a sonicator, a piece of equipment that uses high frequency vibration to assist with dissolving reagent, to re-establish a homogenous solution with no particulate matter. If you are successful, then fill the flask to just a few milliliters below the calibration line. Use your wash bottle of solvent and an eye dropper (also known as a “plastic squeezy baby”) to dilute the solution to the mark. Cap firmly one last time and invert to mix several times. Mixing solutions in a volumetric flask is a key GLP for ensuring a homogenous solution of the intended concentration. Once you no longer need the solution, be sure to rinse the flask clean by using three aliquots of tap water, then one final rinse with DI water and capping off before putting it away.
5.5 Micropipettes
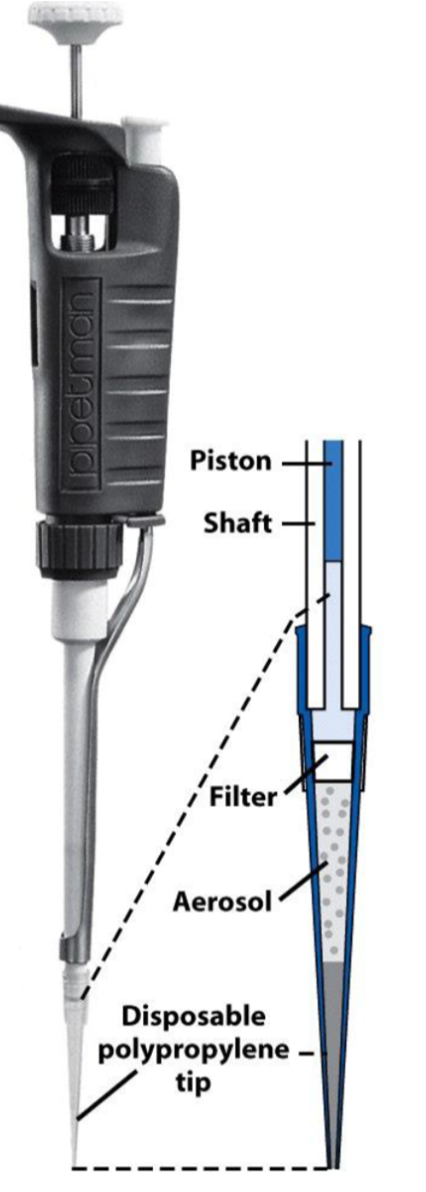
Micropipettes are commercially available in a variety of sizes; some deliver fixed volumes while others are adjustable within a given range. Common volumes for micropipettes in an analytical lab range from about 5 to 2000 µL.
Micropipettes use disposable plastic tips for holding the desired measured volume. To prevent contamination of these tips, insert the clean pipet (not your hand) into a bag or beaker containing the tips and slide it into place. You may need to use a gloved hand to secure the tip from there. Once the disposable tip is firmly in place, the micropipette is ready to use.
Depress the button at the top of the micropipette to “first stop” position and place the tip into the liquid to be transferred. Release the button to pull up the solution and then remove the pipet from the solution. Place the tip into the receiving vessel and then depress the button at the top of the micropipette to the “second stop” position. Micropipettes are a TC type in their volume delivery. The first stop position will deliver most of your solution, but when using a micropipette, you want to be sure you also eject the remaining volume in the tip, which is done by pressing to the second stop.
Some pipets offer a “third stop” position, which ejects the tip. Be sure you have a clear understanding of which stop does which thing for the micropipette in your drawer.
5.6 Burets
Burets are designed to deliver liquids primarily for the purpose of volumetric titrations. They are still widely used in an analytical laboratory and considered a “reference” method for the quantitative determination of a variety of analytes, including the standardization of a solution. A standard 50 mL buret is divided into 1 mL graduations with 0.1 mL subdivisions. The first uncertain digit in a buret measurement is in estimating the 0.02 mL place in-between these subdivisions.
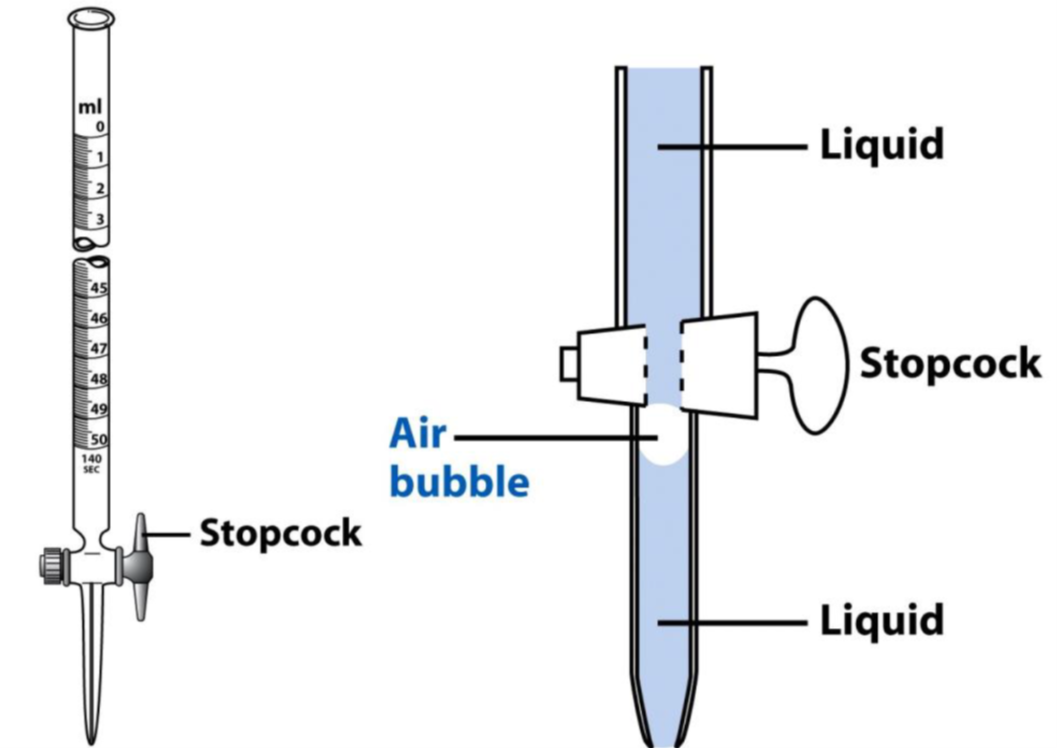
Filling burets:
- Add a few mL of titrant to the buret using a funnel, ensuring the stopcock is closed. Roll the buret using your fingers to internally wet the glass. Dispense the waste to a waste beaker. Do this for a total of three times.
- Fully fill the buret using a funnel, ensuring the stopcock is closed. Run titrant through the stopcock and check for air bubbles lodged just below the stopcock hiding in the tip of the buret (as shown in Figure 4). You need to remove the bubble, which can be done by giving the buret a gentle shake as liquid is leaving the tip.
- Fill the buret one last time, recording the initial volume in your notebook to 0.01 mL.
6. The Analytical Balance
Left-click here to expand ANALYTICAL BALANCE section.
The analytical balance lies at the heart of most measurements made in an analytical chemistry lab. Accurately weighing an object to the nearest 0.1 mg is not a simple process. There are generally three ways you may use a balance in the context of a laboratory exercise. We will get to those details in a minute, but first, understand that in each case, the balance must first be carefully assessed and prepared as described in the appended notes on the analytical balance, located on the course's Canvas page.
In many cases, you will need to weigh out a well-known amount of a reagent, although the exact amount is not critical. For example, suppose you’re instructed to weigh approximately 0.5 grams of a material to the nearest 0.1 mg. You should start this process by first “taring” the balance with the receiving vessel in place. If your receiving vessel is a weighing boat, place the boat in the center of the balance pan, close the doors, and wait for a stable reading. The reading can take as long as 20 seconds to stabilize. When the weight stabilizes, press “TARE”. This sets the zero point of the balance and makes weighing easier. If the weight will not stabilize, check to be sure the beaker or weighing boat is completely cool and dry—the balances are sensitive enough to register the evaporation of water from the glassware. Once the balance has stabilized, transfer about 0.5 g of material. This can be done with a clean dry scoopula or spatula. Any mass that is about the approximate desired value is acceptable (0.45 g to 0.55 g, for example, is an acceptable range). After the reading has stabilized, note down the mass, to the nearest 0.1 mg, of your material. This procedure works well for non-hygroscopic materials that are not prone to static charge.
Note that the “TARE” button can be used at any time to reset the zero point of the balance. The balance has a maximum capacity of 200 g. The weight of any tared object plus the weight of the reagent cannot exceed this amount. For reference, a 500 mL Erlenmeyer flask weighs about 200 g.
If the material to be weighed is hygroscopic, a second approach should be used. In this approach the material to be weighed is placed in a weighing bottle and its ground glass stopper is put in place to protect the material from atmospheric moisture. The material, weighing bottle and stopper are placed on the balance pan. The door is closed and after the balance stabilizes the TARE control is pushed. This sets the balance to zero. Without changing any balance controls, the weighing bottle is removed from the balance and an appropriate amount of material is transferred from the weighing bottle to a beaker or Erlenmeyer flask. This transfer may be accomplished by gently shaking the material into the receiving container. For materials that will not transfer easily a scrupulously clean scoopula may be used, but take care to avoid contamination. The weighing bottle, stopper, and remaining contents are returned to the balance pan and the weight dispensed is noted as a negative weight. As long as none of the material is adhering to the scoopula, this is a very accurate approach. If a little of the material adheres to the scoopula it can be rinsed with solvent into the beaker or Erlenmeyer flask to make the transfer flawless. The scoopula must be carefully dried before further use.
Another situation that is seen in analytical chemistry is the need to weigh an exact amount of material into a beaker. An example of this would be the preparation of a 100 mg/L solution. In this case, 100 mg ± 0.5 mg of solute would be weighed into the beaker. The beaker would be placed on the balance pan and the TARE control would be used to zero the balance. Once zeroed, a scoopula would be used to carefully add 100 mg ± 0.5 mg to the vessel. This requires that the balance door be closed, of course. Plastic weighing boats can be troublesome in this type of work as they are prone to develop static electricity during handling. If this happens your work is compromised because it may be difficult to keep your material in the boat. In addition the electrical fields may spoil your weighing by influencing the balance’s actual response.
While not actually a “weighing step”, a very important aspect of weighing chemicals is making sure your weighed amounts are quantitatively transferred. Let’s consider the 100 mg of solute weighed out in the previous example, and now imagine you need to transfer this solute to a 1 L volumetric flask, in order to make a 100 mg/L solution. The 100 mg of solute must be dissolved in an appropriate solvent and transferred without loss to a one-liter volumetric flask. The solution must be transferred and then the beaker (or weighing boat) must be rinsed three times with fresh solvent. Each rinse must be added to the volumetric flask without loss. This process is the meaning of the term “quantitatively transfer”.
General Rules
- Keep the balance clean at all times. If a reagent is spilled, clean it up immediately. If anything gets under the balance pan, inform the TA.
- Do not handle objects to be weighed with your fingers. Residue from your skin will be left on the object and change the weight.
- Always weigh solid objects in a weighing boat or beaker. If weighing a liquid, use a stoppered bottle to avoid evaporation. Always weigh objects at room temperature. Never place a chemical directly on the pan of the balance.
- Load objects to be weighed in the center of the pan. Close the doors when weighing.
- When transferring materials to be weighed, do this outside the balance to keep the balance clean and avoid spilling.
- At the end of the lab period, turn off the balance, be sure it is clean, close the doors, and replace the dust cover.
7. Gravimetric Calibration of Glassware
Left-click here to expand GRAViMETRIC CALIBRATION OF GLASSWARE section. (includes Table 2)
The balance used for volumetric glassware calibration must itself be calibrated and used with good technique. The temperature of the water must be known accurately enough to eliminate errors from this cause. The precision needed in the temperature measurement depends on the volume of the vessel. For small articles, the nearest 1 °C is usually sufficient, while for a 1-liter flask, it may be necessary to know the temperature to the nearest 0.1 °C.
Care must be exercised in handling the receivers to prevent moisture or grease from the fingers being deposited on the outside. Wiping of the outside with a cloth is sometimes necessary, but in doing so surface moisture may be removed or an electrical charge may be produced on the surface. Usually about 30 minutes are required to restore the surface moisture so that it is in equilibrium with the room. Static electricity will dissipate itself in time, and frequently can be discharged by touching the glass to a clean dry conductor.
Calibration with Water
The estimate of true capacity of a glass vessel at a standard temperature can be obtained from the apparent weight of the water it contains or delivers at any other temperature. To do this, it is necessary to take into account three factors:
- the buoyancy effect of the atmospheric air on the mass measurements,
- the difference in density of water at different temperatures,
- the change in volume of glass vessels with temperature.
Buoyancy Effect
When a vessel is weighed in air, it does not weigh the same as it would in a vacuum (“in vacuo”). This is because the vessel is buoyed up by the air. This is in accordance with the principle of Archimedes, which states that any vessel immersed in a gas (air) is pushed upward by a force equal to the weight of the gas that the vessel displaces.
The weight of the air that is displaced by our glass vessel depends on the density of the air. The density of the air is affected by the altitude at which the experiment is done as well as the current weather. As a result, the apparent weight of a volume of water varies due to changes in air density. In order to make extremely accurate weighings, it is necessary to take into account changes in the air density. The best solution is to calculate the net upward thrust at the time of weighing and add it to the apparent weight. The result is the true weight that would be measured in a vacuum.
For very accurate calibration of volumetric glassware, it is necessary to calculate the buoyancy correction by calculating the actual density of the air at the time that the apparent weight of the vessel is determined. This can be done by measuring the air temperature and the barometric pressure. The impact of the air density on the measurement requires that the buoyancy effect on the vessel and on the standard weight that was used to calibrate the balance be considered. The National Bureau of Standards (NBS) has done the research work on this correction and has provided tables that give the mass correction that must be added to the apparent weight of one mL of water in order to get the weight in vacuo. Their table is in terms of the air temperature and barometric pressure, and the constant is the buoyancy constant.
Change of Density of Water
The volume of water in a vessel can be calculated from the mass of the water in vacuo divided by the density of water at the specified temperature. The density of water at different temperatures has been measured by the NBS and can be used for this volume determination.
Change in Volume of Glass
Glass also changes volume with change of temperature. To find the capacity of a glass vessel at the standard temperature from its capacity at the temperature of weighing, the following formula is used:
| [latex]V_O = V_T \left[ 1 - \alpha \left(T_T - T_O\right)\right][/latex] | (1) |
Where VO = capacity at standard temperature, VT = capacity at the temperature of weighing T, α = cubical coefficient of expansion of the glass, and 1 - α(TT - TO) is the temperature correction of the glass vessel.
While the buoyancy correction provided by the NBS, the water density information provided by the NBS, and the volumetric glass equation above can be used directly to calibrate volumetric glassware, it is more convenient to use the combination of these corrections as supplied by the Kimble Division of Owens Illinois, Inc.
Correction Table for KG-33 Glass When Single-Pan Balances are Used
Table 2 gives the sum of all the corrections - buoyant effect, water density, glass expansion, and apparent mass of built-in weights - for a nominal capacity of 100 mL. For capacities other than 100 mL, the values needed may be obtained by multiplying the values of Table 2 by the appropriate factor.
| Temp. °C | 620 mm Hg | 640 mm Hg | 660 mm Hg | 680 mm Hg | 700 mm Hg | 720 mm Hg | 740 mm Hg | 760 mm Hg | 780 mm Hg | 800 mm Hg |
| 18.5 | .2390 | .2418 | .2446 | .2473 | .2501 | .2529 | .2557 | .2585 | .2613 | .2641 |
| 19.0 | .2480 | .2508 | .2536 | .2564 | .2592 | .2620 | .2647 | .2675 | .2703 | .2731 |
| 19.5 | .2573 | .2601 | .2629 | .2657 | .2685 | .2713 | .2740 | .2768 | .2796 | .2824 |
| 20.0 | .2669 | .2697 | .2725 | .2753 | .2780 | .2808 | .2836 | .2864 | .2892 | .2919 |
| 20.5 | .2768 | .2795 | .2823 | .2851 | .2879 | .2906 | .2934 | .2962 | .2990 | .3017 |
| 21.0 | .2869 | .2897 | .2924 | .2952 | .2990 | .3007 | .3035 | .3063 | .3091 | .3118 |
| 21.5 | .2973 | .3000 | .3028 | .3056 | .3083 | .3111 | .3139 | .3166 | .3194 | .3222 |
| 22.0 | .3079 | .3107 | .3134 | .3162 | .3190 | .3217 | .3245 | .3272 | .3300 | .3328 |
| 22.5 | .3188 | .3216 | .3243 | .3271 | .3298 | .3326 | .3353 | .3381 | .3409 | .3436 |
| 23.0 | .3299 | .3327 | .3354 | .3382 | .3410 | .3437 | .3465 | .3492 | .3520 | .3547 |
| 23.5 | .3413 | .3441 | .3468 | .3496 | .3523 | .3551 | .3578 | .3606 | .3633 | .3661 |
| 24.0 | .3530 | .3557 | .3585 | .3612 | .3640 | .3667 | .3695 | .3722 | .3750 | .3777 |
| 24.5 | .3649 | .3676 | .3704 | .3731 | .3758 | .3786 | .3813 | .3841 | .3868 | .3896 |
| 25.0 | .3770 | .3797 | .3825 | .3852 | .3880 | .3907 | .3934 | .3962 | .3989 | .4017 |
| 25.5 | .3894 | .3921 | .3949 | .3976 | .4003 | .4031 | .4058 | .4085 | .4113 | .4140 |
| 26.0 | .4020 | .4047 | .4075 | .4102 | .4129 | .4157 | .4184 | .4211 | .4239 | .4266 |
| 26.5 | .4149 | .4176 | .4203 | .4231 | .4258 | .4285 | .4312 | .4340 | .4367 | .4394 |
| 27.0 | .4280 | .4307 | .4334 | .4361 | .4389 | .4416 | .4443 | .4470 | .4498 | .4525 |
| 27.5 | .4413 | .4440 | .4467 | .4495 | .4522 | .4549 | .4576 | .4603 | .4631 | .4658 |
| 28.0 | .4549 | .4576 | .4603 | .4630 | .4657 | .4685 | .4712 | .4739 | .4766 | .4793 |
| *Values taken from “The Calibration of Small Volumetric Laboratory Apparatus”, NBS-IR 74-461 and based on a cubical coefficient of expansion of 0.000010 mL/mL/l°C. | ||||||||||
The example below illustrates the use of Table 2.
| Nominal Capacity of vessel (KG-33 Glass) | = 25 mL |
| Temperature of weighing room | = 22.5°C |
| Barometric Pressure of weighing room | = 740 mm Hg |
| Weight recorded on balance before filling receiver | = 28.841 g |
| Weight recorded on balance after filling receiver | = 53.761 g |
| Apparent weight of water at 22.5 °C and 740 mm Hg | = 24.920 g |
| Buoyancy correction for 25 mL at 22.5 °C and 740 mm Hg (0.25 times value in Table 2) |
= 0.084 g |
| Weight of the water in vacuo at 20 °C | = 25.004 g |
| (Built into the correction is the density of water at 20 °C, so that unit grams may be replaced with the unit mL.) |
|
| Volume of vessel at 20 °C and 760 mm Hg | = 25.004 mL |

Lab Concept Video
There is currently no concept lecture video for this lab.
Pre-lab Work
Lab Skills
Review these lab skills videos prior to lab.
click here to hide the video playlist (for printing purpose).
- Preparation of a lab notebook will be a critical skill to develop in this course! Take note on the different sections and ask for detailed feedback on ways you can improve your notebook over the course of the class.
- Be aware of how to use the balance to improve your accuracy and precision.
- There are two major types of glassware, which require different cleaning methods and have different purposes. Improper use of these will impact the accuracy and precision of your measurements throughout the class.
- Make sure you practice with both glassware and the balance.
Extra Resources:
- How To Use an Analytical Balance
- Sample Lab Notebook Pages
- How to Submit PDFs like a Boss
- Making Scientific Plots

Prelaboratory Exercises
There are currently no pre-lab exercises for this lab.

Before You Take The Quiz on Canvas
- Review any videos and appended instructions on Canvas that provide information on how to use the equipment or how to take the prelaboratory quizzes on Canvas.
- Learn the vocabulary of the equipment and procedures described in this manual.
- Understand how to use Table 2 in the background (part 7) to determine the buoyancy correction for a volumetric piece of glassware.
 Experimental
Experimental
In preparing for this week’s lab, familiarize yourself with the care or procedures for preparing the glassware and equipment for use during the semester. Upon arriving to your lab stations, you should review the names of articles in your drawer to confirm you have all the glassware and equipment trusted to your care and also that you know what all the pieces actually are. Once you’ve reviewed the list and confirmed you have what you need, work through the following action items, and check off your understanding where noted with you TA. Your TA should initial your laboratory notebook in the sections noted below.
- Clean glassware and equipment. Note the names of all the glassware and equipment available for your use in your assigned drawer. Notify your TA if you believe you’re missing any items. “CHECK IN” with your TA by signing the class roster to confirm you have all items listed on the equipment list.
- Clean and prepare your desiccator using the procedure described in the background section (part 4).
- Weigh out approximately 5 grams of tris(hydroxymethyl)aminomethane (THAM) into a weigh bottle. Accurately record the mass before drying the reagent. Dry the THAM for about an hour in the 110-degree Celsius oven. Accurately record the mass after drying and when the reagent is cooled. Dried THAM should be stored in your prepared desiccator in preparation for next week’s lab exploring standardization procedures. Write a detailed procedure for how you carried out the drying and storing of this chemical, and have your TA initial your notebook confirming you used the balance correctly.
- Prepare for practice with the volumetric glassware.
- Clean your 120 mL plastic bottle or vial. Use this bottle as receiving container when checking calibration of the pipet and buret.
- Place approximately 1 liter of deionized water in a suitable container and allow it to stand until it has come to room temperature, standing from one laboratory period until the next would be desirable.
Use this water for all of your calibration checks.
- Thoroughly clean a 50 mL buret and a 25 mL pipet.
- Set up your buret in a buret stand and fill with deionized water. After a period of time observe the tip to see if there is any leakage through the stopcock. If there is leakage tighten the retention nut on the stopcock.
- Practice drawing liquids into the pipet using the pipet bulb. REMEMBER ALWAYS USE A PIPET BULB - NEVER USE YOUR MOUTH TO DRAW LIQUIDS INTO A PIPET.
- Practice setting the meniscus on the calibration line.
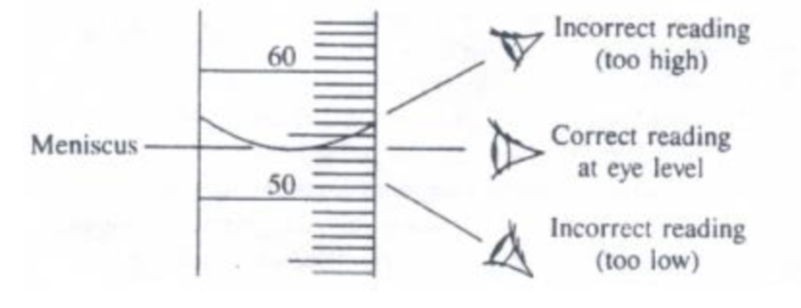 Figure 5: Read the bottom of the meniscus with your eye level to the measurement line. You can use the lines around the buret (in the picture the lines around the 50.00 and 60.00 mL marks) to help align yourself to the level. If you cannot see the back of the line on the buret, then you eye is at eye level to the ring marking that volume.
Figure 5: Read the bottom of the meniscus with your eye level to the measurement line. You can use the lines around the buret (in the picture the lines around the 50.00 and 60.00 mL marks) to help align yourself to the level. If you cannot see the back of the line on the buret, then you eye is at eye level to the ring marking that volume.
- Perform the following calibration checks.
- 25 mL Pipet
- Obtain a “tare weight” of your plastic vial or bottle to the nearest 0.1 mg using the analytical balance. Be sure that the top is on the container. The container must be dry on the outside but need not be dry inside.
- Insert a thermometer into your container of deionized water.
- To ensure that the pipet is at the same temperature as the water, fill the pipet a few times with portions of room temperature water. Discard these rinses.
- Record the temperature of the deionized water.
- Pipet 25 mL of water into the weighed receiver.
- Reweigh the container and water. Be sure the top is placed on the container and that the outside is dry.
- Pipet a second 25 mL portion of water into the weighed receiver. (It is not necessary to empty the container and reweigh for the second portion, just use the second weight of the previous check as the “tare” value.)
- Repeat on a third 25 mL portion.
- Using corrections values from Table 2 in background (part 7), calculate the volume of the pipet at 20 ºC. Obtain the atmospheric pressure from your laboratory instructor.
- The volume measured should be within the tolerances (Table 1 in background part 3) allowed for the class of pipet you have. If not, consult with your laboratory instructor.
- 50 mL Buret
- Insert a thermometer into your 1 L bottle and record the temperature.
- Set up your buret on a buret stand, fill with water from your 1 L bottle. Be sure that the buret tip is filled and there are no air bubbles trapped at the bottom of the stopcock.
- Adjust the level to the 0 mark, if the meniscus setting is not exactly on 0, read the setting and record its value.
- Obtain the “tare” weight of the empty plastic receiving vial or bottle. Weigh to the closest 0.1 mg using an analytical balance.
- Bring the weighed receiver under the buret tip, incline the receiver at a slight angle and touch the tip to the wall of the receiver. Open the stopcock and allow water level to descend to a few mm above the 10 mL mark. Then very slowly set the meniscus at the 10 mL line. If not exactly on the line, read the setting value and record this value to the closest 0.02 mL. Carefully remove the tip from the wall of the receiver; move the receiver away from under the tip and replace the cap on the receiver.
- Weigh the receiver and water.
- Repeat Steps 5 and 6 above for the next 10 mL interval. Continue in 10 mL intervals through the full range of the buret.
- Repeat the calibration of this buret for the same five 10 mL intervals.
- Complete the following calibration table (shown in Table 3 and included in the answer sheet for this lab) using the corrections in Table 2 in background (part 7) to make the necessary calculations.)
- Make a plot of cumulative difference vs. mLs. Keep this in a convenient place because you will want to refer to it during the remainder of this course. (See Figure 2-23 and 2-24 in Harris, Quantitative Chemical Analysis, 10th Edition)
- Using the “reading cards” from the stockroom counter or included at your bench to practice reading the meniscus position of DI water in a buret. When you feel that you have mastered this technique, have your laboratory instructor check your technique and sign your notebook to confirm you’re reading the buret marks and the meniscus placement correctly.
If you haven’t yet, give your TA a clean 125 mL plastic bottle. Your TA should always have an extra bottle on hand, labeled with your name and section number, in order to distribute samples for analysis.
- 25 mL Pipet
| Interval | Buret Readings (mL) | Apparent Volume (mL) | True Weight (g) | True Volume (mL) | (app. V - true V) Difference | Cumulative Difference (mL) |
| Initial | ||||||
| 0 - 10 | ||||||
| 10 - 20 | ||||||
| 20 - 30 | ||||||
| 30 - 40 | ||||||
| 40 - 50 |
Note: “Apparent Volume” is determined by the difference in buret readings. “True Weight” is the balance measurement. The “True Volume” is determined by correcting the true weight by the values in Table 2. “Cumulative Difference” is the sum of the differences.
Post-Lab Work Up

Results/Calculations
Please refer to instructions above and the corresponding answer sheet for any calculations that need to be completed.

Challenge Questions
- Imagine you intend to prepare a 500 mL solution of THAM (the chemical you dried and stored in your desiccator), but you forgot to dry the reagent with the weigh bottle cover removed. Predict the deviation in mass you might expect from the true mass of THAM in your weigh bottle, and explain what type of error (systematic or random) could potentially result from this oversight.

Lab Report Submission Details
Submit your lab report on Canvas as 1 combined PDF file. This submission should include:
- The completed answer sheet.
- Your lab notebook pages associated with this lab.
The grading rubric can be found on Canvas.
References:
- Harris, D. C. & Lucy, C. A. Quantitative Chemical Analysis, 10th ed.; W. H. Freeman: New York, NY, 2020
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please ask your instructor or TA.)
