Spectroscopic Determination of a Mixture
TOC/Help. Click to here expand/hide
Overview
Background
Pre-lab work
Experimental
Post-lab work
![]() For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
Overview
This experiment demonstrates the method of “simultaneous spectrophotometric analysis” to determine the concentrations of multiple absorbing species in a sample.
Learning Objectives
- Apply concepts of absorption spectroscopy and Beer’s Law for the purpose of quantitative analysis of a complex mixture.
- Collect, generate, and manipulate a larger dataset to tease out information of interest.
To cite this lab manual: “Spectroscopic Determination of a Mixture”. A Manual of Experiments for Analytical Chemistry. Department of Chemistry at UW- Madison, Summer 2024.
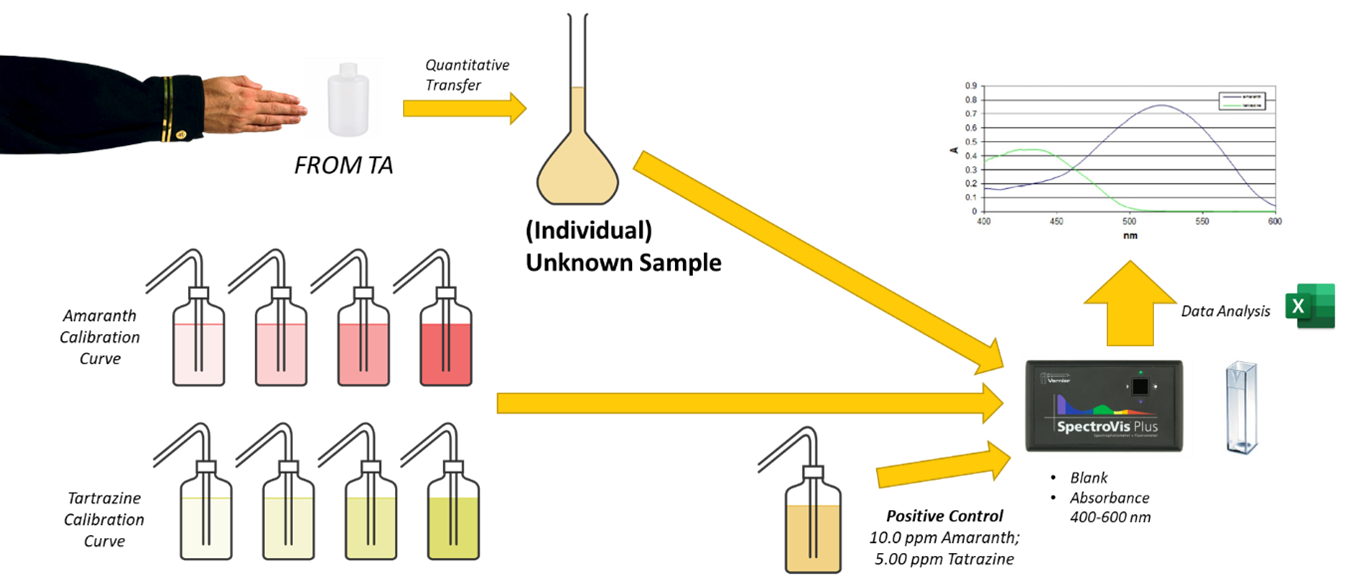
Visual Abstract

Background
Occasionally, a chemist discovers that an analyte is not the only compound in the sample that absorbs at a specific wavelength. This can lead to interference in the analysis due to the presence of additional absorbing compounds. Or perhaps the sample is a complex mixture and a satisfying quantitative result might include reporting the identification and quantitation of several analytes in the mixture. Several methods can address the complication. One approach involves a separation procedure to physically remove the interfering (or secondary) compound(s) from the analyte solution. Alternatively, selecting a different wavelength can resolve the issue if the analyte absorbs well at this new wavelength while the interfering compound does not. However, when the interfering compound is chemically similar to the analyte, separation becomes more difficult. This similarity often results in similar absorption spectra for both compounds. In such situations, simultaneous spectrophotometric analysis can effectively resolve the problem.
Simultaneous spectrophotometric analysis operates on the assumption that each solute absorbs light independently. In this method, the total absorbance of a mixture is the sum of the individual absorbances of the analyte and the interfering (or secondary) compound, provided that the concentration of each solute remains constant. For a two-component mixture, a mathematical representation of the result is summarized as:
| Aλ1 = (εa)λ1bCa + (εb)λ1bCb | (1) |
where Ca is the analyte concentration, Cb is the concentration of the interfering compound, (εa)λ1 is the molar absorptivity of the analyte at λ1, and (εb)λ1is the molar absorptivity of the interference at λ1.
Equation (1) contains two unknowns: Ca and Cb. In this scenario, it is necessary to find a second independent equation, which we fulfill by measuring the absorbance of the mixture at a second wavelength. One may write:
| Aλ2 = (εa)λ2bCa + (εb)λ2bCb | (2) |
This system of equations is solvable for Ca and Cb. The only requirement is to select two wavelengths that correspond to the absorption spectra/maxima of the compounds of interest. The use of the two equations requires knowledge of (εa)λ1 and (εa)λ2, (εb)λ1 and (εb)λ2. These values can be determined by measuring the absorbance at λ1 and λ2 of a solution of known concentration of the analyte. Plotting the absorbance at λ1 and λ2 of a set of standard solutions of known concentration of both analytes, one can calculate (εb)λ1 and (εb)λ2. These four ε values can then be substituted into equations (1) and (2). The resulting equations can then be used to find Ca and Cb based on Aλ1 and Aλ2 for the mixture.
Consider an example: Vanillylmandelic acid and p-hydroxymandelic acid are two urinary phenolic compounds. The vanillylmandelic acid concentration is of diagnostic significance; p-hydroxymandelic acid is an interfering compound. The two compounds are difficult to separate, but the mixture of the two can be extracted from the urine. The absorbance of the two compounds is then determined by absorption spectroscopy at two wavelengths. The vanillylmandelic acid concentration is found by the simultaneous analysis approach.
Before beginning such an analysis, it’s important to explore the nature of the spectrophotometric data. In our experiment, the analyte is tartrazine, and our interference is amaranth. Amaranth and tartrazine are commercial dyes used as food coloring.
To use Beer’s law for this quantitative analysis, we will need to obtain the absorption spectra of known concentrations of each dye at two different wavelengths. From these data, Beer’s law plots of absorbance vs. wavelength (using equations (1) and (2)) should prove a linear relationship exists between the two variables. The slopes of these four lines will yield the ε’b values for tartrazine and amaranth at the chosen wavelengths. (These values are ε’b values because the concentrations are in ppm or mg/L rather than moles/liter, so we use a “prime” to note the difference in units.) Another necessary element in this type of analysis is to show experimentally that these compounds absorb independently of one another. With this in mind, let’s move to the experimental portion of the experiment.

Lab Concept Video
click here to hide the video (for printing purpose).
Write down observations or notes from the video in your lab notebook.
Pre-lab Work

Lab Skills
Review these lab skills videos prior to lab.
click here to hide the video playlist (for printing purpose).
Key Takeaways
- Spectrophotometers are powerful tools to measure the concentration of a species by the amount of light they absorb at a specific wavelength.
- Excel is a useful tool to work up the resulting data.
Extra Resources:

Prelaboratory Exercises
In your lab notebook, include a detailed purpose and procedure (including the list of equipment you will need), as well as responses and calculations to solve the following problems.
- Suppose you have two control samples: One is 10 ppm vanillylmandelic acid and the other 30 ppm p-hydroxymandelic acid. You measure absorbances at 0.22 and 0.68 respectively. Predict the resulting absorbance if you mixed 5 mL of each solution together and measured the absorbance of the resulting mixture.
- Why is the absorptivity coefficient presented with a prime (‘) notation?

Before You Take The Quiz on Canvas
- Review the additive nature of absorbance for a solution containing more than one independently absorbing species.
- Describe the proper method of obtaining quantitative absorbance data using one cuvette.
- Be able to calculate the concentration of each absorbing component in a solution given a set of raw data, which would include absorbance of the solution at two wavelengths, the molar absorptivity coefficient, and the path length of the cell.

Experimental
In this experiment, you will measure the absorbance of the following solutions from 400 to 600 nm. To make the measurements, you should use the SAME cuvette for both blanking as well as for measuring absorbance. Be sure you orient the cuvette similarly each time to minimize differences in reflectance of the light beam from irregularities in the cuvette.
| Tartrazine standards | Amaranth standards |
| 2.50 ppm | 2.50 ppm |
| 5.00 ppm | 5.00 ppm |
| 7.50 ppm | 10.0 ppm |
| 10.0 ppm | 20.0 ppm |
| Positive Control – 10.0 ppm amaranth, 5.00 ppm tartrazine | |
| Sample of unknown concentration | |
- There are a total of nine solution standards prepared for you. Collect absorbance spectra for all eight standard solutions using the LabQuest spectrophotometers. Be sure to also collect the positive control spectrum. The blank for all spectra is deionized water. Export these files as text files to more fully analyze using Excel.
- Unknown sample. Give your instructor a clean, labeled 125 mL plastic bottle. Quantitatively transfer the sample to a 100 mL volumetric flask and dilute to the mark with deionized water. Mix well. Collect the spectra of your unknown sample.
Post-Lab Work Up

Results/Calculations
Use a spreadsheet to prepare a table of the absorbance of each of the ten solutions versus wavelength. Then:
- Make a plot of the absorbance of each of the tartrazine standards versus wavelength. Organize these four spectra on a single full-page plot.
- Repeat 1 but use the amaranth data.
- Prepare a plot of the absorbance of the 10.0 ppm tartrazine standard versus wavelength and of the absorbance of the 20.0 ppm amaranth standard versus wavelength. Overlay these spectra on a single plot.
- Sum the absorbance values at every wavelength for the 10.0 ppm amaranth standard and the 5.0 ppm tartrazine standard in a new column in the Excel spreadsheet. Make a plot of this sum versus wavelength. Plot absorbance values versus wavelength for the positive control on the same chart as the summed spectra. Organize these two spectra as a single plot.
- Make a full-page plot of absorbance at 460 nm versus concentration of the tartrazine standards. Also do this at 425 and 480 nm. Be sure to include zero absorbance at zero concentration as a data point. Print these three calibration curves on the same full-page printout. Plot your data as points only, then add a trend line to each data set to show the linear functionality.
- Make a full-page plot of the absorbance at 460 nm versus concentration of the amaranth standards. Also do this at 425 and 480 nm. Be sure to include zero absorbance at zero concentration as a data point. Print these three calibration curves on the same full-page printout.
Be sure to include ALL PLOTS in the submission you hand in to Canvas.
Fill out the answer sheet for this experiment completely. Answer the following post-lab questions.
- Consider the plots from (1) and (2) from above.
- What is the λmax of tartrazine?
- What is the λmax of amaranth?
- Is the λmax a function of concentration? Explain your answer.
- Does the value of ε vary with wavelength? Explain your answer.
- Consider the absorption spectrum of 10.0 ppm tartrazine and the spectrum of 20.0 ppm amaranth. Using this plot, explain why tartrazine is the analyte and amaranth is the interference. Why is the converse not true?
- Consider the calibration curves that you have prepared for tartrazine and amaranth.
- Does Beer’s Law seem to apply to this system?
- Explain why the wavelengths of 425 nm and 480 nm are the best choice for the analysis of the unknown sample. Could other wavelengths be used that would work effectively for the analysis?
- Report your calculated values of ε’b for both amaranth and tartrazine at your analysis wavelengths.
- Describe the change that would occur in the appearance of your Beer’s Law plot if you used a cell with a longer path length for your measurements.
- What is the concentration of amaranth and tartrazine in your sample of unknown concentration? Include the absorption spectrum of your unknown.

Challenge Questions
Challenge questions are designed to make you think deeper about the concepts you learned in this lab. There may be multiple answers to these questions! Any honest effort at answering the questions will be rewarded.
- You are tasked with performing a Quality Assurance analysis for Jell-O’s lemon jello product and have just prepared a sample for analysis. When measuring the absorbance, the spectrometer reads 1.4 A. Your standard curve range is very linear between 0.2 and 0.9 A, with the concentration range for tartrazine being 1 and 20 ppm, respectively.
- Estimate the concentration given the set of data above. Identify what questions a customer or your boss might have if you reported this as the result in a final report.
- What GLP processes for making spectroscopic measurements do you need to improve to make the QA report acceptable?
- What are your next steps in the analysis?

Lab Report Submission Details
Submit your lab report on Canvas as 1 combined PDF file. This submission should include:
- The completed answer sheet.
- Your lab notebook pages associated with this lab, which should include answers to the post-lab questions and challenge questions.
The grading rubric can be found on Canvas.
References:
- Harris, D. C. & Lucy, C. A. Quantitative Chemical Analysis, 10th ed.; W. H. Freeman: New York, NY, 2020
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please ask your instructor or TA.)
