Preparation of a Standard NaOH Solution
TOC/Help. Click here to expand/hide
Overview
Background
Pre-lab work
Experimental
Post-lab work
![]() For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
For help before or during the lab, contact your instructors and TAs (detailed contact information are found on Canvas).
Overview
You’ll continue the practice of identifying a colorimetric titration endpoint for the purpose of standardizing a solution. Save the standardized NaOH solution from today’s lab to use for subsequent experiments in the lab schedule.
Learning Objectives
- Develop and hone skills with the analytical balance and volumetric glassware by using a primary standard to accurately standardize a solution to four significant figures.
- Apply basic statistics to a data set to report a result as an average value with a correctly determined standard deviation.
- Develop proficiency in techniques, such as, using the analytical balance, performing quantitative transfers, safe and accurate transfer of liquids and reagents, and reading the meniscus formed in a variety of volumetric glassware.
To cite this lab manual: “Preparation of a Standard NaOH Solution”. A Manual of Experiments for Analytical Chemistry. Department of Chemistry at UW- Madison, Spring 2025.
Visual Abstract
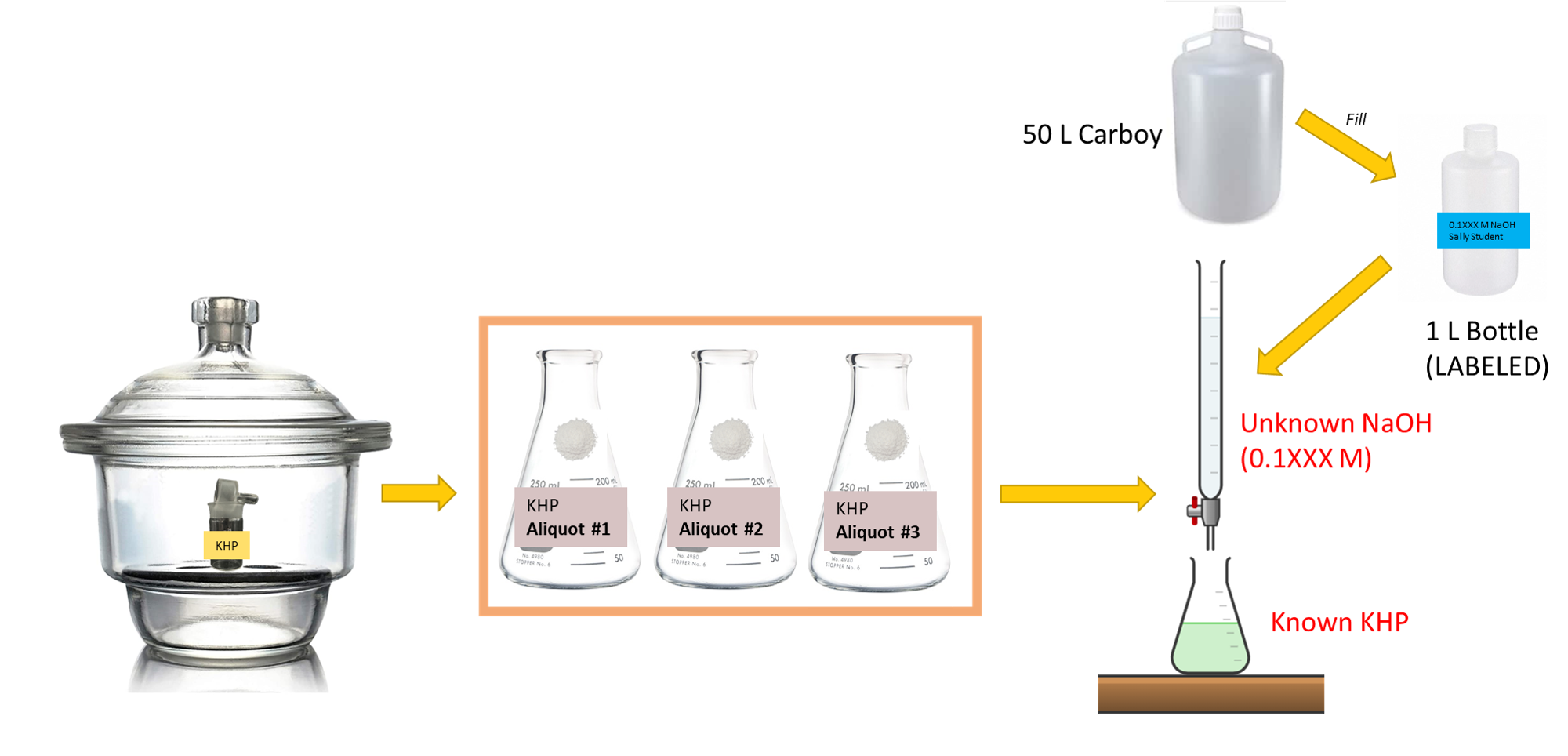

Background
Chemistry of the Titration
A standard solution is a solution of well-defined concentration. Sometimes standard solutions are commercially available, and it is more efficient and dependable to buy them from a commercial source. Other times, standard solutions are unavailable or are too expensive, and we have to standardize them ourselves. Standardization is the process of determining the exact concentration of reagent in your solution. In the case of acids and bases, we usually standardize the acid/base by titrating the analyte and a primary standard. A primary standard is a reagent that is chemically pure and stable and reacts with the analyte in a known mole ratio to a definitive endpoint.
Analytical quality sodium hydroxide contains small amounts of impurities, including water and carbonate. It is important to correct for these known contaminants and to standardize the solution to get a precise concentration. The easiest way to correct for carbonate is to first prepare a highly concentrated solution of sodium hydroxide (~50 w/v %). Carbonate salts are generally insoluble in such highly alkaline solutions. The solution of desired concentration can be prepared by diluting the highly concentrated solution. After dilution, one then standardizes the solution to confirm its exact concentration.
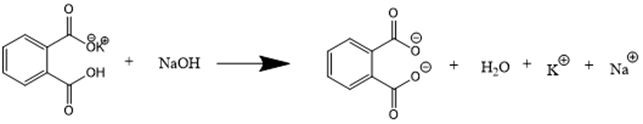
Potassium hydrogen phthalate (KHP) is widely used for standardizing strong bases due to its excellent qualities as a primary standard. It is readily available in very pure form, has a high molecular weight, is non-hygroscopic (does not absorb water from the air), and remains stable when dried at 100-110 °C. KHP reacts with NaOH in a one-to-one mole ratio, as shown below:

Lab Concept Video
click here to hide the video (for printing purpose).
Write down your observations or notes from the video in your lab notebook.
Pre-lab Work

Lab Skills
Review these lab skills videos prior to lab.
click here to hide the video playlist (for printing purpose).
Key Takeaways
- Titrations take a lot of patience and practice to hit the end point correctly.
- By using volumetric glassware, we can improve the accuracy and precision of our measurements.

Prelaboratory Exercises
The following questions should be completed in your laboratory notebook before you take the quiz, and especially before you start lab. You will need the answers to the questions below to start the laboratory experiment!
- Write out the chemical reaction for the titration of KHP with NaOH. Identify the mole ratio of the starting reagents.
- You will use a 50 ml buret to titrate with. NaOH will be placed in the buret. You should use the optimum range of the buret for the titration, which is between 25 – 40 ml.
- If using more than 40 ml, you will run the risk of having to refill the buret for the titration. This adds a significant amount of error to your measurement.
- If using less than 10 ml, the reagents may be too concentrated, leading to passing the endpoint of the titration hastily. Also, by using less than 10 ml, the tolerance label for the buret no longer applies, since the number is meant for the entire volume.
Choose an optimum volume of NaOH that you would like to use for the titration. Then, calculate the grams of KHP you must weigh out that will completely react with the NaOH present in that volume.
- Based on your results to question 2, calculate how much KHP you would need to dry to complete this experiment- bear in mind you will need to do at least 3 titrations and the reagent may lose as much as half its weight while it dries in the oven and desiccator.

Before You Take The Quiz on Canvas
- Learn the chemistry and stoichiometry of the titration reactions.
- Be able to calculate how much KHP should be used for the standardization given the approximate concentration of NaOH and a target end point volume.
- Be able to calculate the precise concentration of NaOH given a set of titration data (mass of KHP used, and the volume required to reach the end point).

Experimental
Each student should carry out at least three titrations of a sample collected from a class carboy. SAVE YOUR NaOH.
- Place 4-5 g of pure potassium acid phthalate in a clean weighing bottle and dry in the oven at 100-110 °C for 1-2 hours. Cool and store in your desiccator.
- You may have already done this in a previous lab period.
- Obtain about 1 L of ~0.1 M NaOH solution in a polyethylene bottle.
- Accurately weigh three portions of the calculated amount of KHP (from your pre-lab questions!) into three clean 250 mL Erlenmeyer flasks. Dissolve each in approximately 50 mL of room temperature deionized water that has been boiled or air-sparged to remove the CO2.
- For each prepared Erlenmeyer flask, add 2 drops of phenolphthalein indicator and titrate each with the 0.1 M NaOH solution.
- Calculate the molarity of the NaOH solution based on the results from each titration. Then, report your final number in terms of a mean and standard deviation.
Do NOT discard the NaOH solution. You will be using it in the next experiment.
Post-Lab Work Up

Results/Calculations
Please refer to instructions above for any calculations that need to be completed.

Challenge Questions
Challenge questions are designed to make you think deeper about the concepts you learned in this lab. There may be multiple answers to these questions! Any honest effort at answering the questions will be rewarded.
- From the pre-lab videos and your experience in the lab, rationalize what would happen if you used bromocresol green to observe the endpoint rather than phenolphthalein.
- Propose a modification to the procedure for swapping the titrant with the aliquot volume. In other words, what procedural considerations would you need to be aware of if you put the NaOH in the Erlenmeyer flask and then filled the buret with the KHP solution?

Lab Report Submission Details
Submit your lab report on Canvas as 1 combined PDF. This submission should include:
- The completed answer sheet.
- Your lab notebook pages associated with this lab, which should include answers to the Challenge Questions.
The grading rubric can be found on Canvas.
References:
- Harris, D. C. & Lucy, C. A. Quantitative Chemical Analysis, 10th ed.; W. H. Freeman: New York, NY, 2020
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please ask your instructor or TA.)
