In each pre-class assignment, there is a Podia question that requires numerical, text, and/or image-based response. Each pre-class Podia question is based on the pre-class material and working through the pre-class material will help you formulate your response. Consider the problem and write down/draw out your solution in your class notebook. These questions are designed to hone your skills so that you can analyze and solve mastery problems you will encounter throughout the course.
Two days before the next whole-class session, the pre-class Podia question will become visible in Podia, where you can click on the prompt and submit your solution. [For example, the Podia question for a Friday pre-class assignment will be open for submission by Wednesday evening.] Your submission will only be visible to yourself and your instructor.
You may find Podia sign-in instructions for
- 109-1 (MWF 12:05 PM – 12:55 PM) here
- 109-2 (MWF 2:25 PM – 3:15 PM) here
- 109-3 and 109-4 (MWF 8:50 AM – 9:40 AM) here (note: 109-3 and 109-4 share a single Podia community)
Submissions for pre-class Podia activities are due 1 hour before the start of your whole-class. Be sure to submit your response before the due time. If your response shows that you have made full effort to answer the question as well as you could, regardless of whether your answer was correct or not, you will receive full credit for your response.
Day 2 Pre-Class Podia Activity
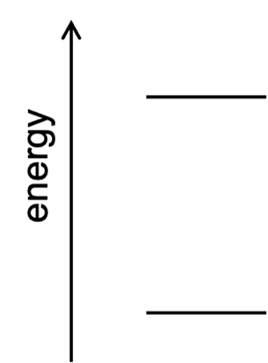 Consider the element sodium, whose emission spectrum exhibits an intense yellow line at 589 nm. Construct an energy-level diagram like that at right to represent how the energy of the sodium atom changes during this emission event. Label which energy level corresponds to the sodium atom’s ground state and which corresponds to the sodium atom’s excited state.
Consider the element sodium, whose emission spectrum exhibits an intense yellow line at 589 nm. Construct an energy-level diagram like that at right to represent how the energy of the sodium atom changes during this emission event. Label which energy level corresponds to the sodium atom’s ground state and which corresponds to the sodium atom’s excited state.
During this emission event, by what amount (in J) does the energy of the sodium atom change? Label this energy on your energy-level diagram.
Answer the questions below using using Coulomb’s law reasoning. For each answer, briefly identify how your model supports or depicts your claim.
- Does the magnitude of the sodium atom energy increase or decrease?
- Does the sodium atom energy become more or less negative?
- How do these sodium atom energy changes connect with the notion that atoms “lose” energy during emission events?
Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. We appreciate your comments. 🙂 (Note that we cannot answer questions via the google form. If you have a question, please post it on Piazza.)

