1 Overview
The enzyme Nitrogenase is one of the most important biological molecules without it, life as we know it would not exist on earth. Nitrogenase mediates the process of Biological Nitrogen fFxation, which is the biological conversion of the Nitrogen atoms in dinitrogen (N2) to Ammonia. Fixation refers to combination of Nitrogen atoms in N2 with other molecules, which in this case is Hydrogen.
Fixation is essential as formation of molecules that are essential, such as proteins and nucleic acids, and many others requires relatively large amounts of fixed Nitrogen.
Nitrogenase is remarkable because dinitrogen is one of the most stable molecules known the atoms of, and two are held together by a triple bond. Triple bonds are in general, the strongest types of bonds. The N2 triple bond is especially strong because it occurs between two of the same atoms. Which means all the electron orbitals are exactly at the same energy levels. So the bond results in a molecule with symmetrical charge distribution across the two atoms. (Fig. 1.1) This electronic structure of N to makes it inert and it doesn’t react readily with other molecules.
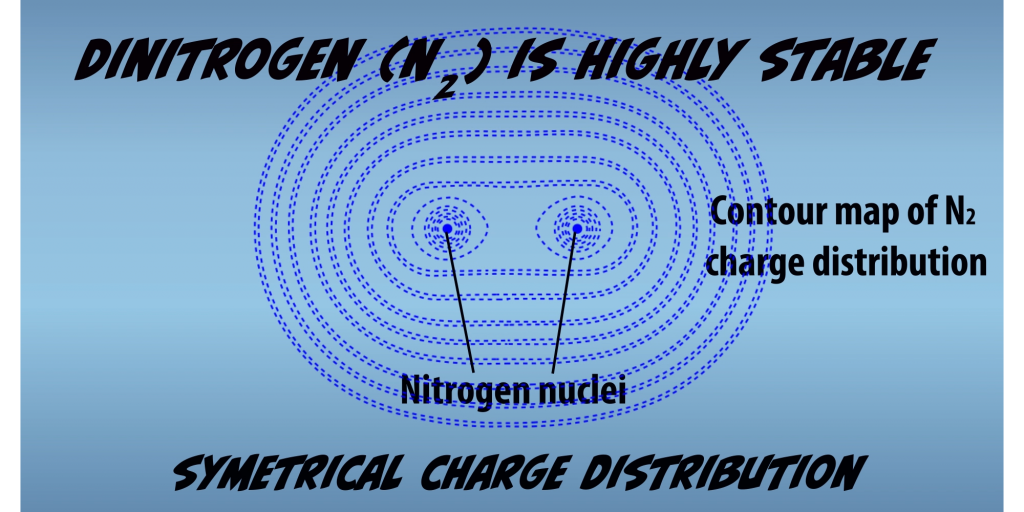
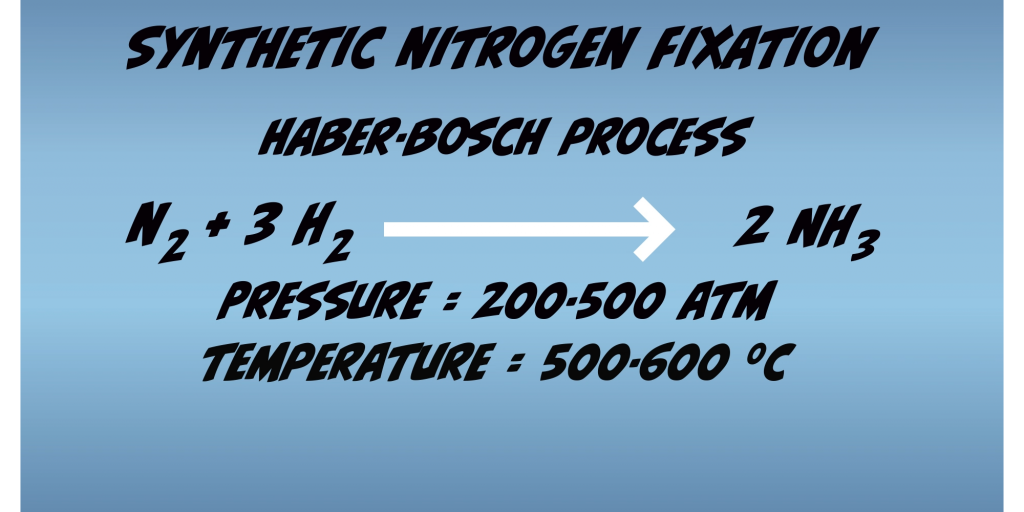
Fixation thus requires significant energetic input to force N2 into interactions in which it otherwise would not participate. Humans have been able to mimic this accomplishment and developed a method for synthetic nitrogen fixation. This technique is the Haber-Bosch process (Fig. 1.2) and requires extremely harsh conditions: high pressures of 200 to 500 atmospheres, which equates to 1.5 to 3.6 tons per square inch and high temperatures of 500 to 600 degrees centigrade, conditions which no organism could survive. In contrast, Nitrogenase accomplishes the same process within living microbial cells at ambient temperatures and pressures of the Earth’s biosphere.
Prior to 1920, Biological Nitrogen Fixation was the only process that existed which could provide fixed Nitrogen in amounts sufficient to support life on earth. Since then, use of Nitrogen fertilizers derived from synthetic fixation has steadily grown in agronomic production and has been a key factor supporting the rapid growth of the human population that has occurred over the past hundred years (Fig. 1.3).
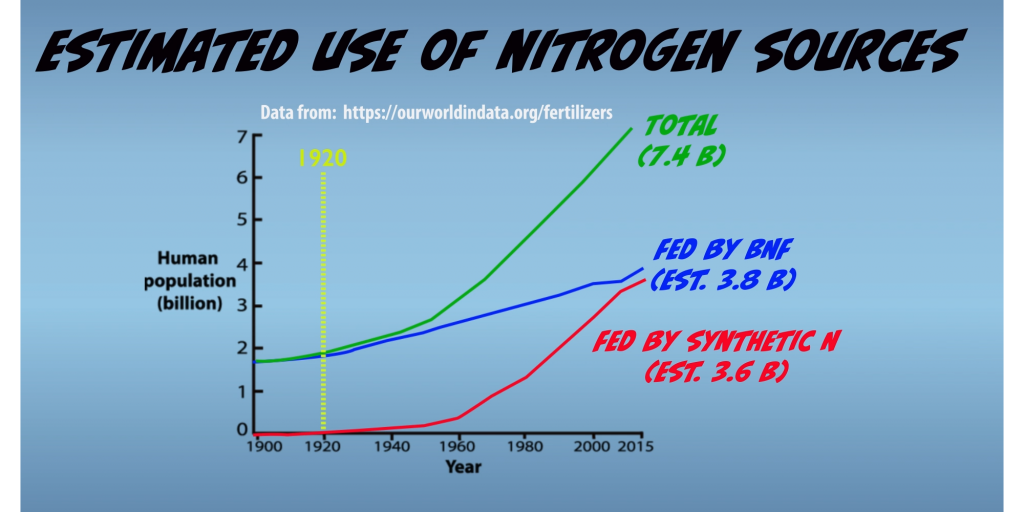
Nevertheless, Biological Nitrogen Fixation remains as the primary source of fixed nitrogen in agroecosystems that are estimated support lives of about 3.8 billion people or about half of the world’s population. Moreover, Biological Nitrogen Fixation also remains the primary source of fixed Nitrogen for all natural ecosystems, which includes the world’s ocean.
So, if you’re interested in learning more about this incredibly important enzyme, join me in this presentation where we’ll take an in-depth look at the mechanisms Nitrogenase employees to carry out its essential function, including what the latest research has revealed at the molecular level about how Nitrogenase functions.
