6 What is the role of Molybdenum in Nitrogenase function?
Before leaving the M-cluster, one last thing to talk about is Molybdenum and examine the role that this somewhat unusual atom may play in Nitrogenase function. The short explanation is that exact functions are unknown. But hypotheses have been developed by comparisons of Nitrogenase isoforms.
Isoforms are enzyme variants that catalyze the same reaction. Nitrogenase has three isoforms that differ in the M-Cluster metal content. One of these is the protein that we’ve been focused on so far, Molybdenum Nitrogenase. Another isozyme has vanadium replacing Molybdenum, and is called Vanadium Nitrogenase. The third variant has Iron in place of Molybdenum and is referred to as Iron Nitrogenase or the all Iron Nitrogenase (Fig. 6.1).
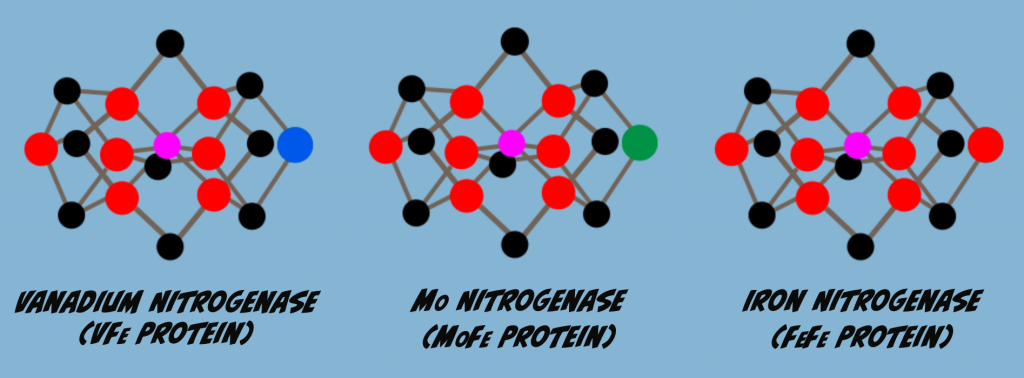
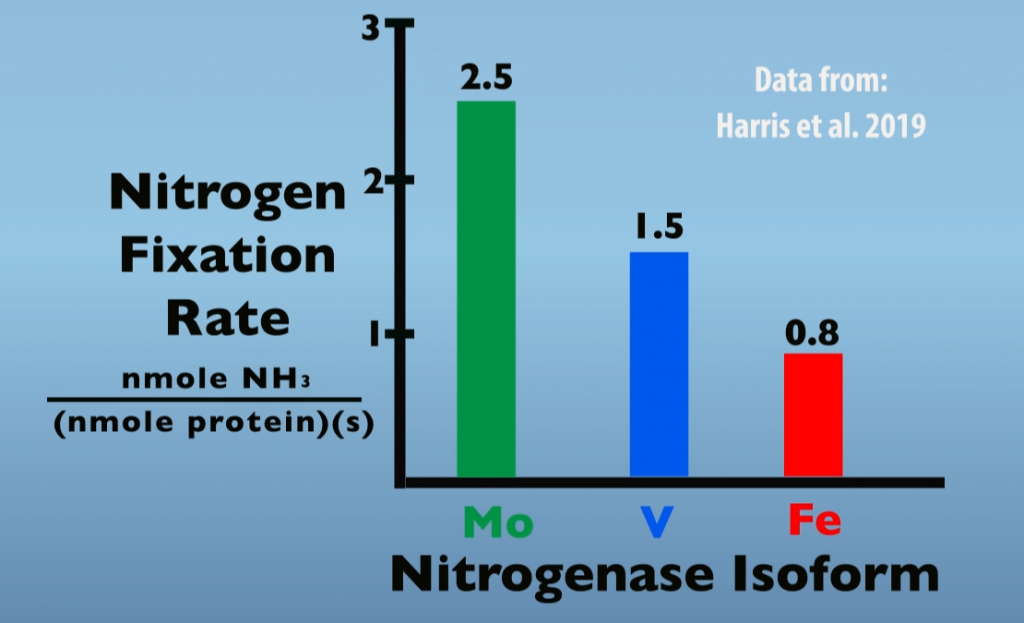
When Molybdenum is limiting or absent, other isozymes will be produced in place of Molybdenum Nitrogenase. Thus, Vanadium- and Iron-Nitrogenase are referred to as alternate Nitrogenases. Perhaps the main reason for preferential production of Molybdenum Nitrogenase is that it is the isozyme most effective in nitrogen fixation. For example, the graph below (Fig. 6.2) illustrates rates of Nitrogen Fixation for each of the three Nitrogenase isoforms. In this test, Molybdenum Nitrogenase was about 1.7 times more effective than Vanadium Nitrogenase and three times more effective than Iron Nitrogenase.
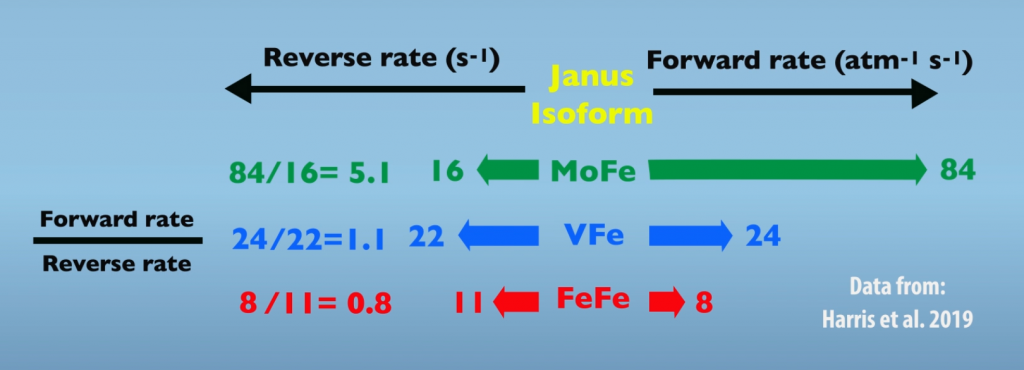
All three Nitrogenases are hypothesized to function by the same mechanism. So, differences in efficiency may be traced to the Janus intermediate. Specifically, we can compare the performance of isozymes in the forward and reverse reactions (Fig. 6.3). Looking first at the reverse reaction, which is hydride protonation leading to relaxation, the rates between isozymes do not differ greatly. However, with the forward reaction, Molybdenum Nitrogenase is much faster than the other isozymes. So, the key difference in efficiency is that the Molybdenum Nitrogenase is much more effective in the forward reaction than it is in the reverse. These differences between the isozymes can be summarized by looking at the ratios of the forward to reverse reactions. In this comparison, Molybdenum Nitrogenase is roughly five times more reactive in nitrogen fixation than the other two isoforms.
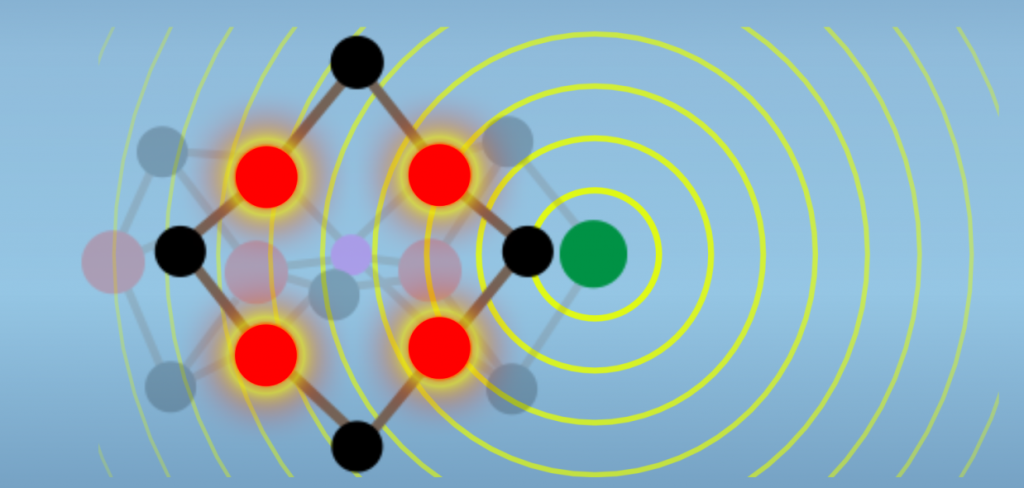
The mechanisms by which Molybdenum may enhance reactivity aren’t established. However, recent data show that there is electronic interaction between the Molybdenum atom and the Iron atoms of the reactive face (Fig. 6.4). Thus, the hypothesis is that Molybdenum acts to tune the M-cluster as a whole, in a manner that enhances reactivity with N2.