2 Characteristics of the proteins that comprise Nitrogenase
We’ll begin by examining some basic characteristics of the proteins that comprise Nitrogenase. These are structural features that are key to understanding how Nitrogenase functions and are aspects that are foundational to later parts of this video, where we’ll dive deeper into the mechanisms of nitrogen fixation.
Nitrogenase is composed of two proteins (Fig. 2.1). One of these is called the Iron Protein (Fe Protein) and the other is termed the Molybdenum Iron Protein (MoFe Protein). The Fe Protein is composed of two subunits of a single protein. The MoFe Protein is composed of two different proteins. Each present in two copies. These are called the alpha subunit and the beta subunit.
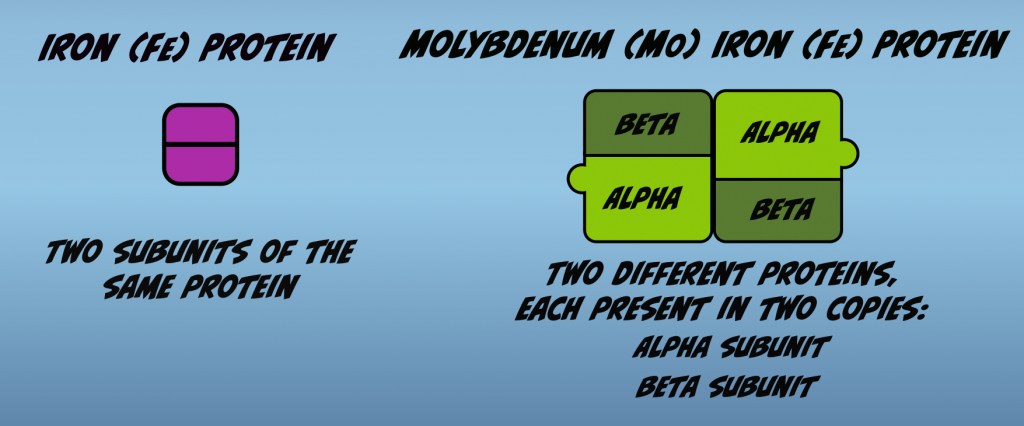
Each of the proteins of Nitrogenase has unique roles and both are required for biological nitrogen fixation. The MoFe Protein binds and reduces nitrogen. The Fe Protein transfers electrons to the molybdenum iron protein that are used in nitrogen reduction to ammonia. The Fe Protein is also the part of Nitrogenase to which Adenosine Triphosphate (ATP) binds.
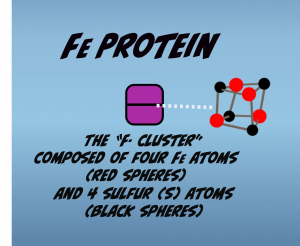
Central to Nitrogenase function are metal clusters that mediate the electron flow that underlies nitrogen fixation. The iron protein contains one metal cluster called the “F-Cluster.” It’s composed of four iron atoms and four sulfur atoms (Fig. 2.2). The F-Cluster is located at the interface of the two subunits and holds an electron that is transferred to the MoFe Protein
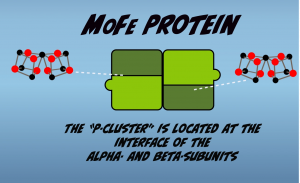
The MoFe Protein contains two different types of metal clusters. One of the these is called the “P-Cluster”, which is also composed of iron and sulfur, and is located at the interface of the alpha and beta subunits (Fig. 2.3). The P-Cluster serves as an intermediary in the electron transfer process occurring in nitrogen fixation, as it accepts an electron from the Fe Protein F-cluster and transfers this electron on to the other metal cluster in the MoFe Protein, called the “M-Cluster.”
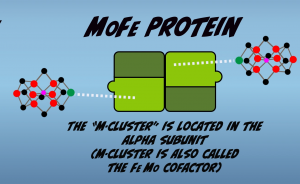
The M-Cluster is located in the alpha subunit (Fig. 2.4). Like the other clusters is also composed of iron and sulfur, but it’s structurally more complex and also contains an atom of Molybdenum and an atom of Carbon.
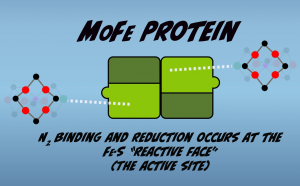
The M-Cluster contains the active site of the enzyme and Nitrogen binding and reduction occurs at the region termed the “Iron-Sulfur Reactive Face” (Fig. 2.5).
For Nitrogenase to work, Fe Protein and the MoFe Protein must join to form the Nitrogenase complex. The complete Nitrogenase complex consists of two Fe Proteins docked to one MoFe Protein, forming a symmetric dimer (Fig. 2.6).
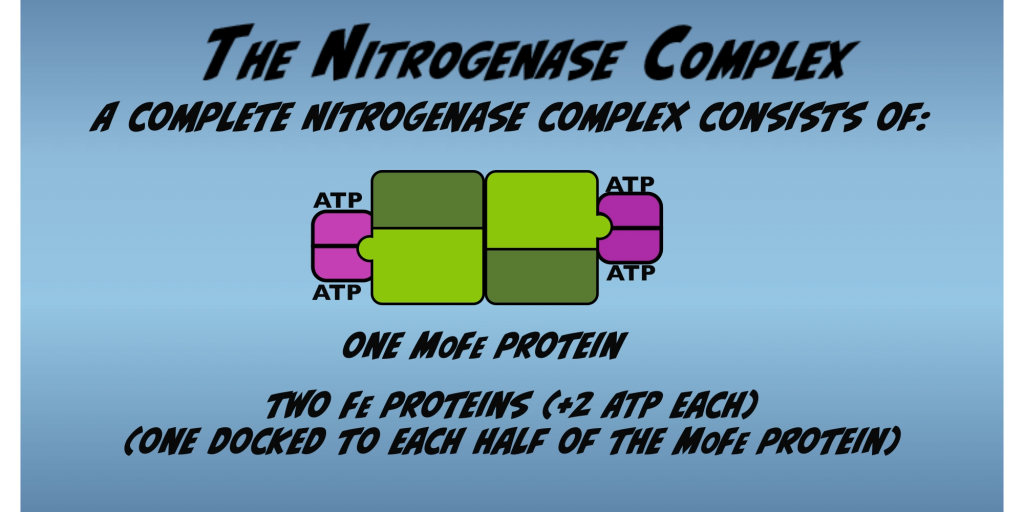
Nitrogen fixation occurs in each half of these concurrently. And there’s evidence that mechanical communication occurs across the protein. So that activity in one half affects that occurring in the other. However, the two halves function identically. So, for simplicity, all the following diagrams and figures, will focus on one half at of the complex for illustration of activity.
