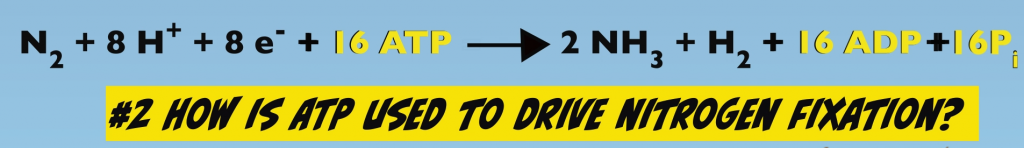
3 The Nitrogenase chemical equation: “Mysteries” of Nitrogenase function
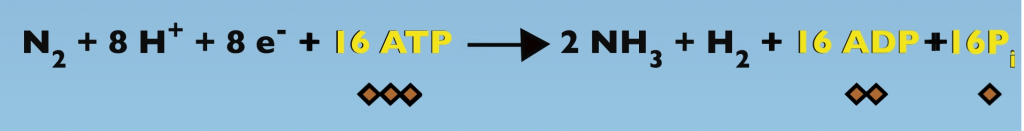
Biological Nitrogen Fixation expressed as a chemical reaction provides a good starting point in a discussion about Nitrogenase function (Fig. 3.1).

The reactants in this process are: one N2, eight protons, eight electrons and 16 adenosine triphosphate, which yields two ammonia molecules, one molecule of hydrogen gas, 16 ADP and 16 phosphates.
The chemical equation provides a description of essential features of Biological Nitrogen Fixation, but it’s a relatively high level, sometimes referred to as the view from 30,000 feet and key mechanistic details on Nitrogenase function are not apparent.
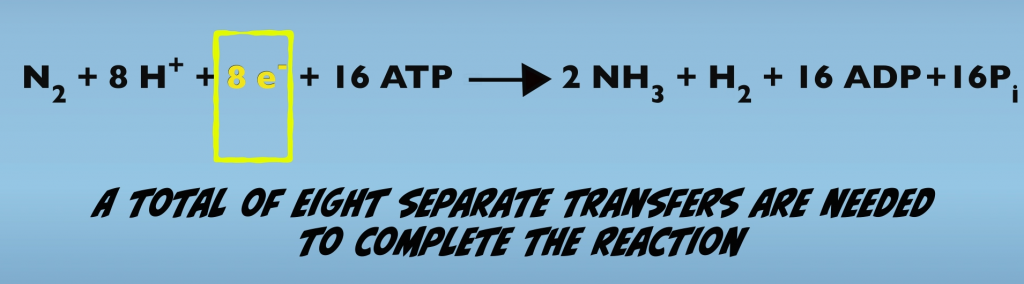
For example, this equation is actually a summary of eight separate interactions of the Fe Protein and the MoFe Protein (Fig. 3.2). Each of these interactions results in the formation of the Nitrogenase complex, the transfer of one electron and consumption of two ATP.
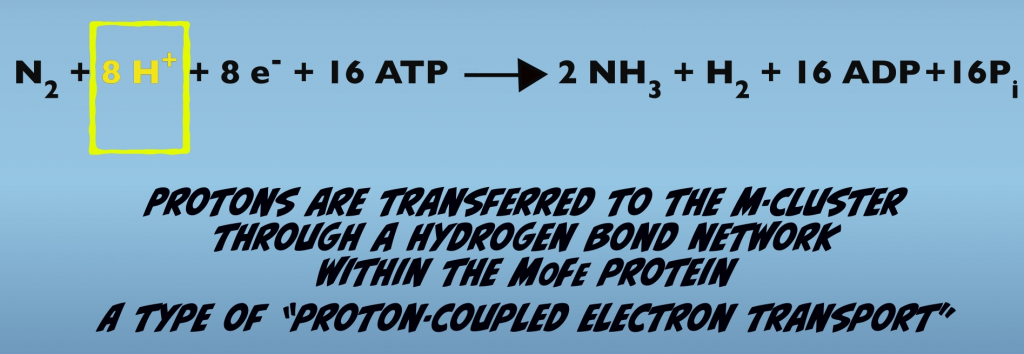
Also, while the Fe Protein serves as the source of electrons listed in the equation, it’s not the source of protons. Instead, protons originate from a hydrogen bond network within the MoFe Protein itself. This parallel, but separate transfer of electrons and protons is an example of a process term proton-coupled electron transport, which also occurs in other biological processes, such as respiration and photosynthesis (Fig. 3.3).
From the 30,000 foot view of the chemical equation, two apparent mysteries of Biological Nitrogen Fixation emerge. First, why does Nitrogenase produce hydrogen gas?
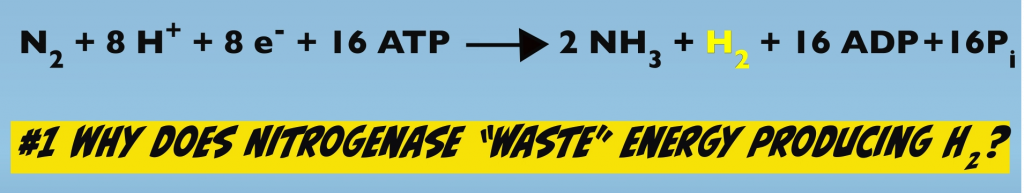
It’s an apparent waste of energy because, as we just noted, each electron transfer consumes two ATP. If you do the math, two electron transfers times two ATP per transfer equals a total of four ATP that are used to produce one molecule of hydrogen gas. So that’s four ATP out of the 16 ATP total that are used in the process, which is an apparent waste of 25% of the total energy that’s consumed.
The other mystery concerns how the chemical energy present in ATP is used to drive Biological Nitrogen Fixation. The chemical reaction simply shows hydrolysis of the phosphate bond, which releases energy (Figs. 3.5A, 3.5B).
However, the equation provides no hint as to how energy released by hydrolysis is captured and utilized in Nitrogen fixation.
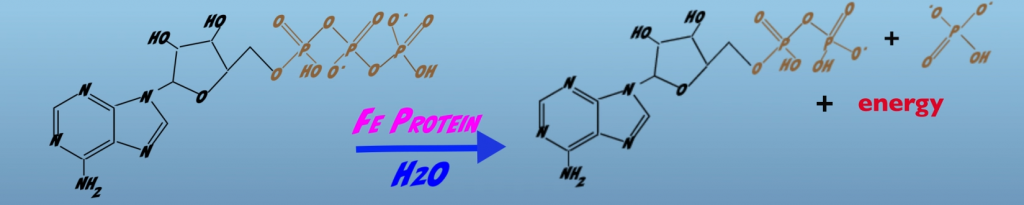
Moreover, besides phosphate bond hydrolysis, there are other mechanisms by which ATP can provide an energetic input into biological processes, which are not expressed in a chemical equation. Most notably, and important for Nitrogenase, are changes in a protein structure that occur with ATP binds to a protein. In this case, the altered shape induced by ATP-binding brings the protein to an elevated energy state (Figs. 3.6A, B).


One last point before leaving the discussion of ATP is to introduce the diamond symbols that I’ll use to represent ATP and its hydrolysis products later in this presentation (Fig. 3.7). Each diamond represents a phosphate group. So, ATP with three phosphates is represented by three diamonds. ADP is indicated by two diamonds and one diamond is used for a single phosphate.