6 Electrophilic Addition of HX to Alkynes
Alkynes undergo electrophilic addition in much the same manner as alkenes. Therefore, the understanding we acquired thus far, involving Hammond’s postulate and relative carbocation stability, can also be used to predict the major product of addition reactions involving alkynes.
For example, when 1-hexyne is reacted with one equivalent of HBr (1-hexyne and HBr are present at 1:1 molar ratio), two competing pathways are possible:

The carbocation intermediates formed here are vinyl carbocations, where the nominally empty 2p orbital is on a sp hybridized carbon. Despite this difference, the reasoning regarding carbocation relative energy still applies: more alkyl substituents lead to a lower energy cation. Here, one pathway (top, magenta) forms a 2° vinyl carbocation, whereas the other pathway (bottom, blue) forms a higher energy 1° vinyl carbocation. By Hammond’s postulate, we can reason that the lower energy carbocation intermediate indicate a lower energy transition state, and therefore a faster reaction pathway. Thus, the major product for this reaction is 2-bromo-1-hexene (top, magenta). In other words, this reaction also obeys Markovnikov’s rule.
Notice that the product here is also an alkene, which can itself undergo HX addition. Therefore, when excess reagent is present, the addition reaction can occur twice consecutively. The regioselectivity of the HX addition to the haloalkene is also governed by the relative energy of the carbocation intermediate.
For an example, consider the addition reaction of 2-butyne with excess (>2 equivalent) HBr.
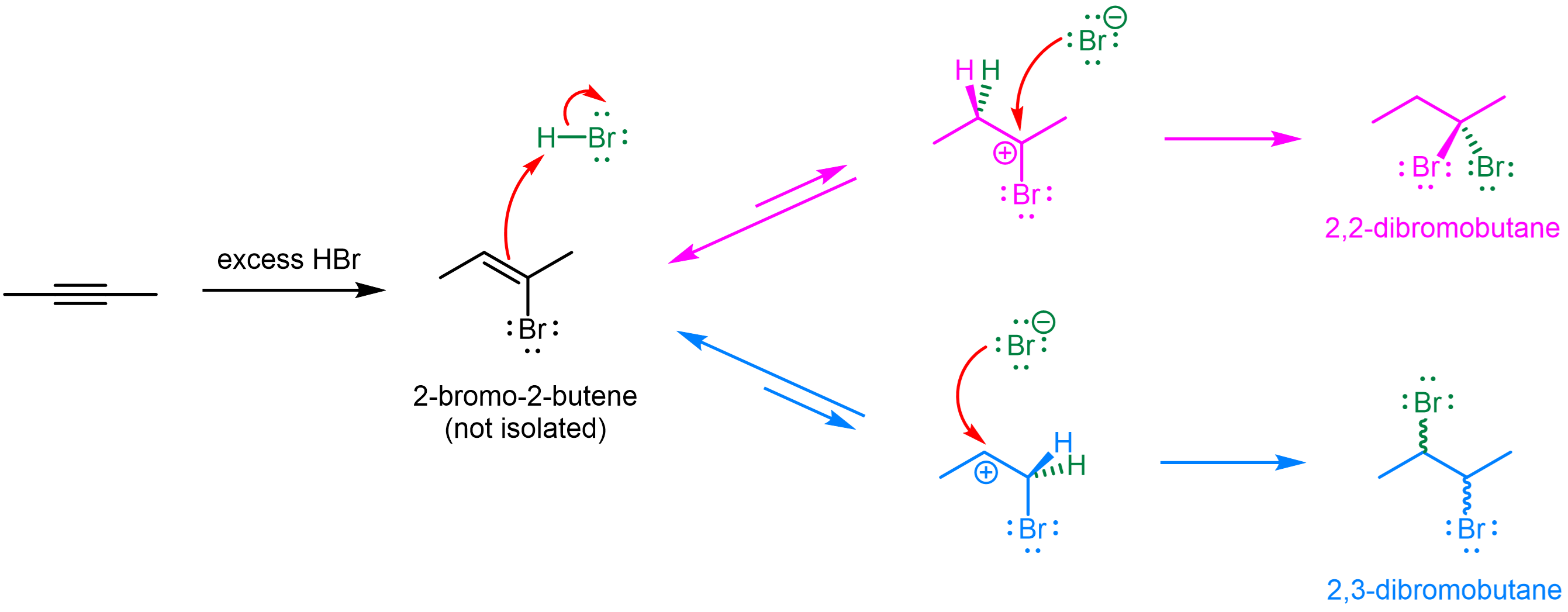
Upon the addition of the first HBr, a 2-bromo-2-butene product is formed, which go on to react with another HBr. Two competing pathways are possible here, and upon a quick examination, it may seem that these two carbocation would be similar in energy—they both have two alkyl substituents. Moreover, the presence of bromine, an electron-withdrawing group[link to acid-base content on induction effect], in these two carbocation raises their energy relative to a carbocation without a halogen. And by that reasoning, one may expect the top (magenta) carbocation to be higher in energy because bromine is closer to the electron-deficient carbon.
However, looking closer, we can see that the top (magenta) carbocation would actually be lower in energy due to π conjugation. (Note that there is no π conjugation in the bottom (blue) carbocation because there is a sp3 carbon in between the sp2 carbon and bromine.)
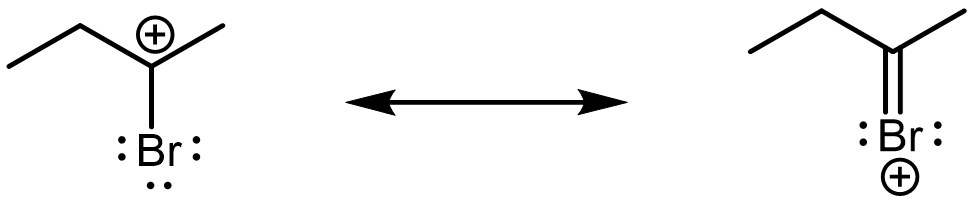
Therefore, with lower energy carbocation intermediate and hence faster reaction rate, the major product for this reaction is 2,2-dibromobutane (top, magenta).
The inductive effect does play a role in this reaction, because the second HX addition is usually slower than the first one. This is the reason why when only 1 equivalent of HBr is added, the haloalkene product can be isolated; in other words, the reaction can be stopped at the first addition. However, the electron-withdrawing halogen is present in both pathways during the second HX addition (Figure 2), and the difference due to the EWG location is not as big as the additional presence of π conjugation. And it is the fact that the energy-raising induction effect is partially offset by the energy-lowering π conjugation that leads to the observed regioselectivity.
Exercise 1: Addition to Alkynes
Provide the missing reagent(s) for the transformation below. For any reagent(s) that must be added sequentially, be sure to number each separate step.
