7 Hydration of Alkenes
In another variation of electrophilic addition reaction involving alkenes, let us consider the reaction where an alcohol or an ether can be formed from an alkene substrate.
The hydration reaction involves the addition of a water molecule to an alkene in the presence of a strong acid. The strong acid, such as H2SO4 or HNO3, dissociates in water and provides H3O+ as the electrophile that initiates the reaction with alkene. For an example, consider the following reaction,
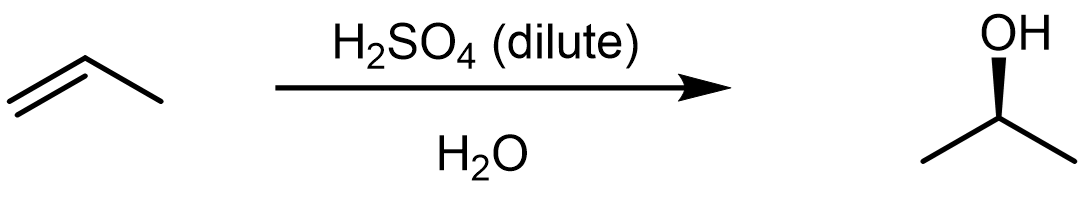
which proceeds via a mechanism that is quite similar to the HX addition reactions.

In the first step, a strong acid, H3O+, reacts with the alkene π bond, yielding a 2° carbocation. This is the rate-limiting step, and the formation of the lower energy carbocation intermediate leads to the observed regioselectivity in this reaction.
In the second step, a nucleophile, H2O, reacts with the electrophilic carbocation, forming a C-O σ bond. Compared to the X– in HX additions, a neutral water molecule is not as good of a nucleophile. But the carbocation is a very reactive species, and it will readily react with a water molecule to form a protonated alcohol. Note that it is not hydroxide, OH–, that is the nucleophile here—there is no OH– present in an acidic solution! [link to earlier content on H2O acid/base reaction]
In the third step, the protonated alcohol reacts in a Brönsted-Lowry acid-base reaction with a water molecule to form the final alcohol product, as well as reforming the H3O+ acid.
Note that in the overall reaction written above, H2O is written under the reaction arrow rather than listed as a reactant. Water is serving two roles here, it is both the solvent in which the reaction occurs and a reactant that is consumed by the reaction. On the other hand, there is no net consumption of the acid because it is reformed during the last step of the reaction.
If the above reaction occurred in an alcohol solvent rather than water,
![]()
then an ether product would form via an analogous mechanism:
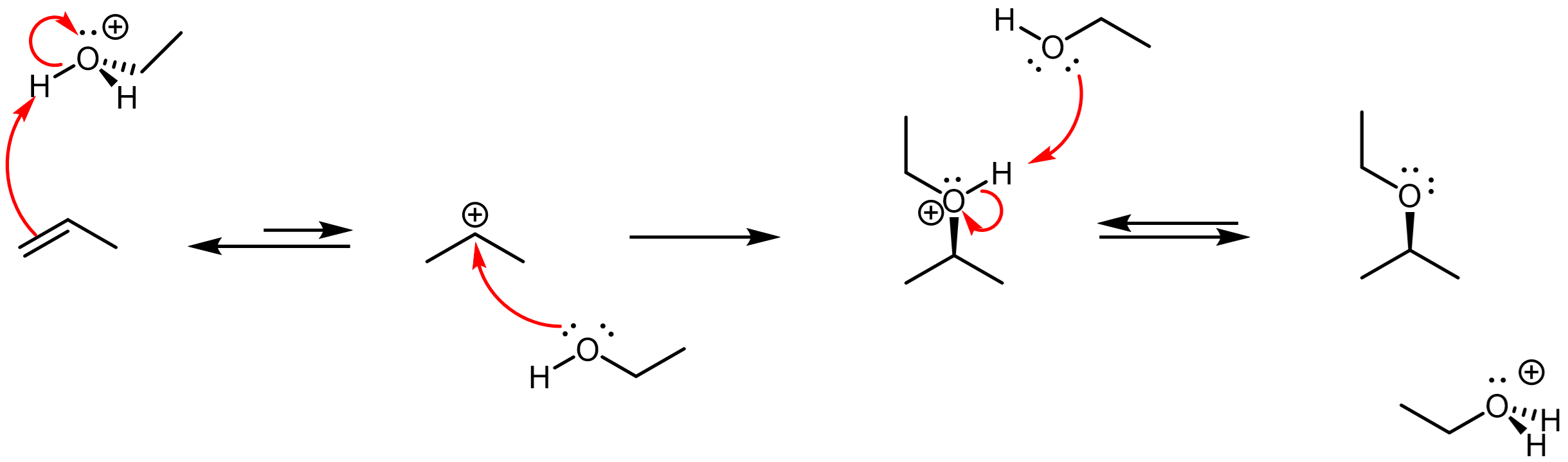
dfd
