2 Alkene Isomers and Nomenclature
Stereoisomers
Unlike a σ bond[link to earlier content on conformational isomer], a π bond is not cylindrically symmetric about the bonding axis, but rather has electron densities above and below the bonding plane.
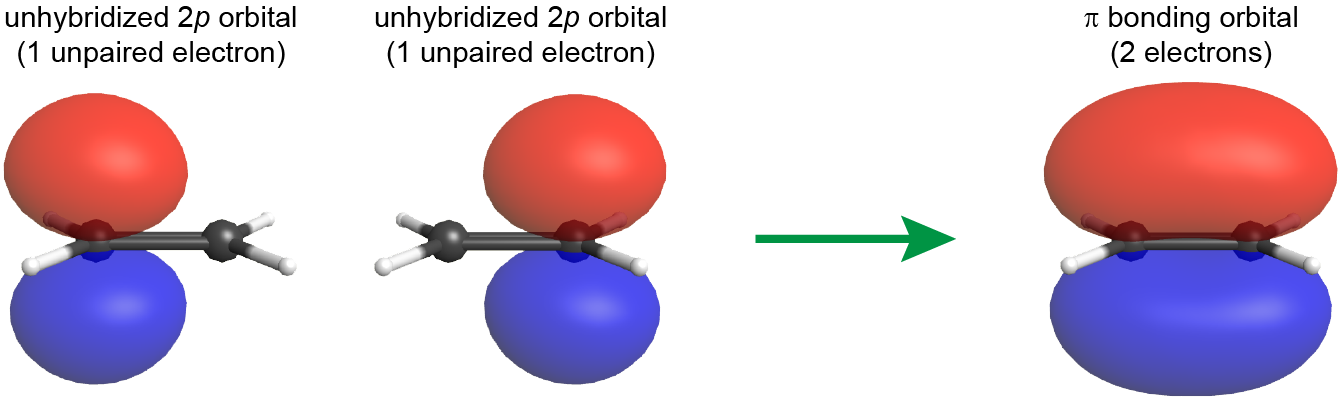
If one carbon atom is rotated 90° relative to the other carbon atom, then the side-by-side overlap of the 2p atomic orbitals would no longer be possible, and the π bond is effectively broken.

Therefore, the internal rotation about a C=C double bond involves crossing over a transition state that essentially involves breaking the π bond while leaving the σ bond intact, and such a transition state typically has a barrier height of ~250 kJ/mol. Such a high energetic demand means that, unlike a C-C single bond, a C=C double bond cannot freely rotate at room temperature.
This rigid character gives rise to stereoisomers, molecules that have the same molecular formula and atomic connectivity, but differ in the orientation of the atoms in 3D space.
For an example, consider the isomers of butene (alkene isomers with the formula C4H8):
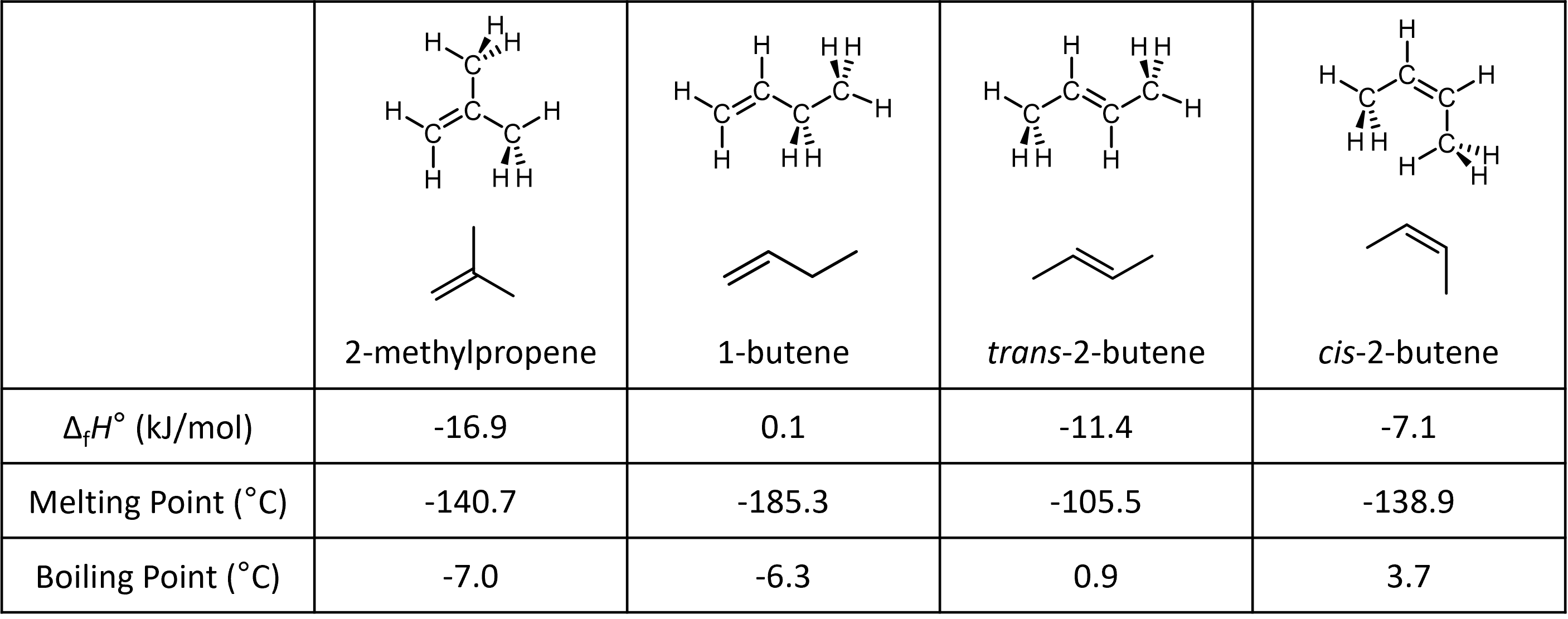
While 1-methylpropene, 1-butene and 2-butene are constitutional isomers[link to earlier content], the two 2-butene isomers are stereoisomers—they differ from each other in the configuration around the double bond. In trans-2-butene, the two methyl groups are on the opposite side of the double bond; in cis-2-butene, they are on the same side. Trans-2-butene is a distinct compound from cis-2-butene, with differing physical properties such as melting point and boiling point.
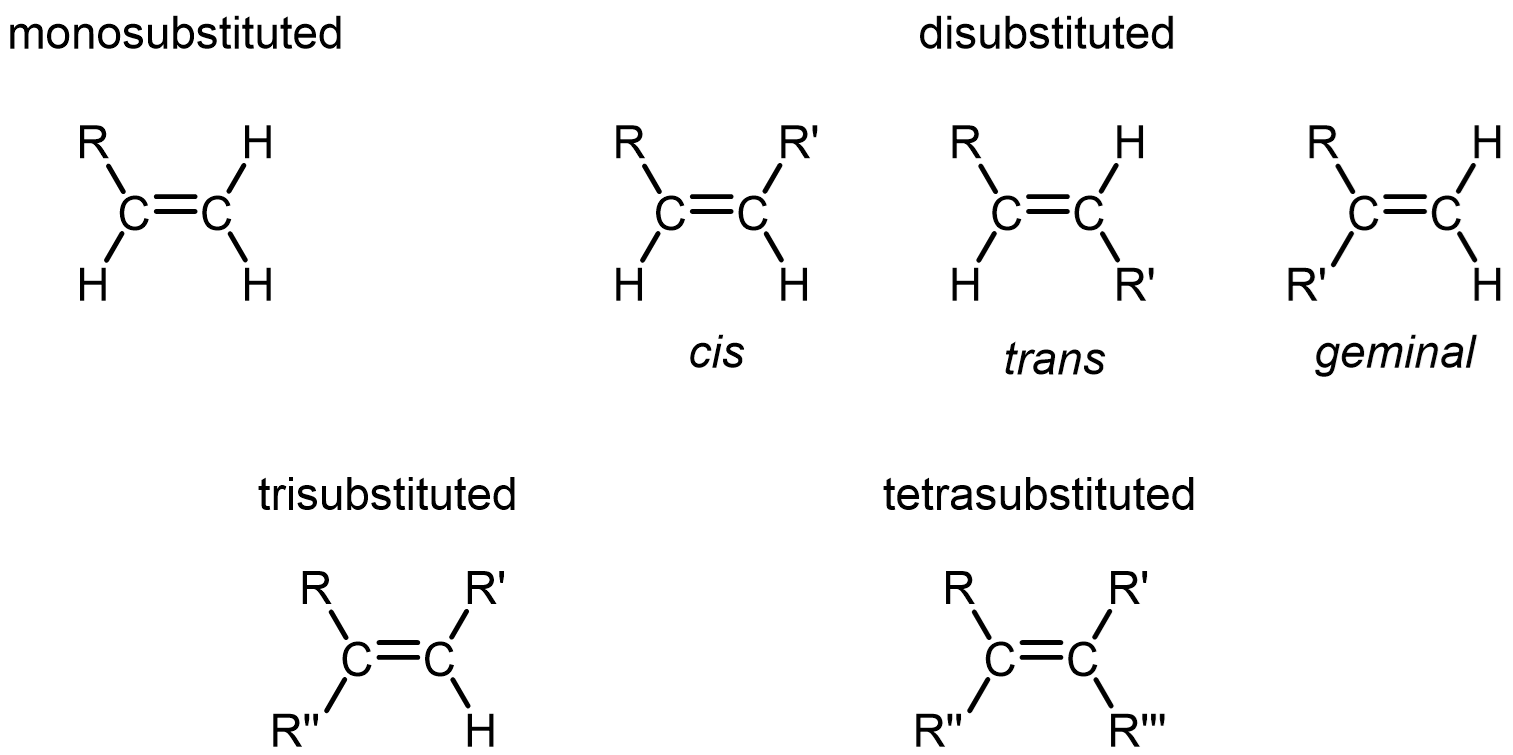
Note that only alkenes with two different groups at each end of a double bond have stereoisomers. For example, the 1-butene in Figure 3 has no cis or trans isomers. This is because, while one of its doubly-bonded carbon has two different groups (-H and -CH2CH3), the other doubly-bonded carbon does not (it has two -H groups).
In general, monosubstituted and geminal disubstituted alkenes have no stereoisomers, while tri- and tetrasubstituted alkenes may or may not have stereoisomers depending on the nature of the R groups.
Exercise 1: Isomers 1
Determine the unsaturation number of C5H10, and draw all the possible constitutional isomers and stereoisomers of C5H10. Organize them by functional group.
Answer the question (write and draw) in your notebook, then left-click here.
C5H10 has an unsaturation number of 1: containing either one double bond (alkenes) or one ring (cycloalkanes).

Exercise 2: Isomer relative energies
Which isomer of cyclohexene is known and commonly used, cis or trans? Provide a justification as to why the other isomer has not been isolated.
Answer the question (write and draw) in your notebook, then left-click here.
cis-cyclohexene is known and can be constructed without a lot of strain.

trans-cyclohexene must distort the double-bond, otherwise C4 and C5 cannot connect and form a bond.

(Click on the link above or build the molecules with a model kit to see the differences between these two isomers.)
Exercise 3: Isomers 2
Consider the constitutional isomers and stereoisomers of C3H4Cl2.
- What is the unsaturation number or degrees of unsaturation?
Write in your notebook, then left-click here.
C3H4Cl2 has an unsaturation number of 1. - Draw all the species that contain at least one approximately sp2 hybridized carbon atom.
Draw in your notebook, then left-click here.

- Draw all the species that contain no approximately sp2 hybridized carbon atoms.
Draw in your notebook, then left-click here.
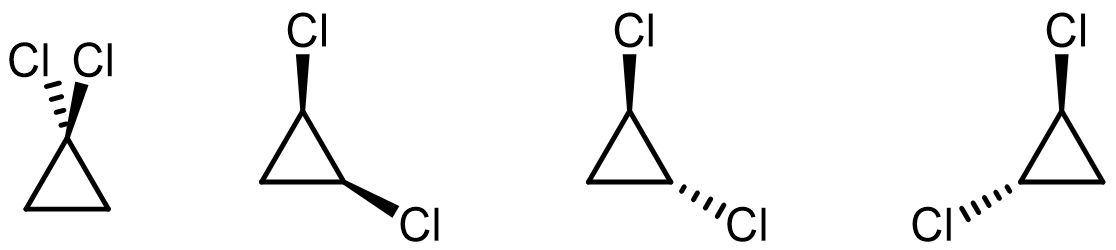
Note that the three structures at the right involves a type of stereoisomers called enantiomers. We will learn more about them later on in the semester. [add clarification notes about what students need to know/able to do at this point?]
Alkene Nomenclature
For straight chain alkenes, the IUPAC nomenclature has the same basic rules as alkanes, except the -ane suffix is changed to -ene.
- Find the longest carbon chain that contains both carbons of the double bond.
- Start numbering from the end of the parent chain which gives the lowest possible number to the double bond.
- If the double bond is equidistant from both ends of the parent chain, number from the end which gives the substituents the lowest possible number.
- The double bond in cycloalkenes do not need to be numbered because it is understood that they are in the 1 position.
- Place the location number of the double bond directly before the parent name. [or before the -ene suffix, per newest IUPAC?]
- The location number indicates the position of the first carbon of the double bond.
- The presence of multiple double bonds is indicated by using the appropriate suffix such as -diene, -triene, etc. Each of the multiple bonds receives a location number.
- Add substituents and their position to the alkene as prefixes. Remember substituents are written in alphabetical order.
Cahn–Ingold–Prelog (CIP) Sequence Rules
Named after Robert Sidney Cahn, Christopher Kelk Ingold, and Vladimir Prelog, CIP system is a standard process to completely and unequivocally name a stereoisomer of a molecule.
In the case of alkenes, CIP is used to assign an (E) or (Z) notation, which is an extension of the cis–trans notation. (The cis–trans notation describes relative stereochemistry: cis indicates that the substituents are on the same side of some reference, while trans conveys that they are on opposing (transverse) sides. For a double bond containing three or four substituents, such naming system quickly becomes ambiguous.)
Using the CIP rules, each substituent on a double bond is assigned a priority.
- Atoms that are directly attached to the double-bonded carbons are assigned priority based on decreasing atomic number (highest atomic number = highest priority).
- For example, fluorine (F) has higher priority than carbon (C).
- If two groups have the same atomic number, consider atomic mass (higher atomic mass = higher priority).
- For example, deuterium (2H) has higher priority than hydrogen (1H)
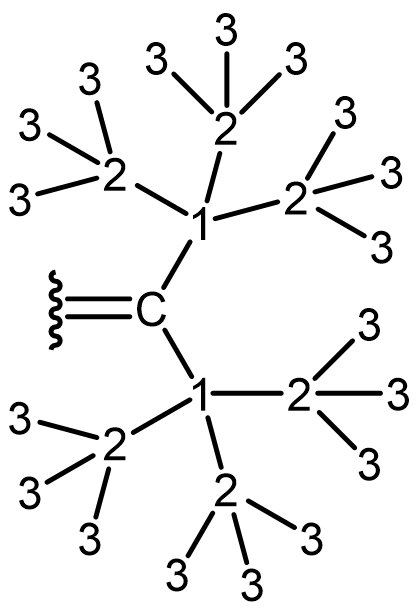 If two groups have the same atomic number and mass, use the next attached atoms (move along the chain to a different atom) until there is a difference in priority.
If two groups have the same atomic number and mass, use the next attached atoms (move along the chain to a different atom) until there is a difference in priority.
- If two groups have the same atomic number and mass and lie in the same position along the chain, but one of them is doubly bonded, then count that atom twice.
- For example, –CH=O has higher priority than -CH2OH.
- If two groups have the same atomic number and mass and lie in the same position along the chain, but one of them is doubly bonded, then count that atom twice.
If the two groups of higher priority are on opposite sides of the double bond, the bond is assigned the configuration “E” (from the German word entgegen, meaning opposite). If the two groups of higher priority are on the same side of the double bond, the bond is assigned the configuration “Z” (from the German word zusammen, meaning together).

Let us consider the 2-butene example again. For the trans-2-butene, the left carbon of the double bond (shown in red) has either an H or a C (in -CH3) bonded to it. Per rule 1 above, C has higher priority. The right carbon (shown in blue) has either an H or a C bonded to it, and again C has higher priority. Hence, the two “higher priority” groups are on opposite side, one up one down, and this is the (E)-2-butene molecule.
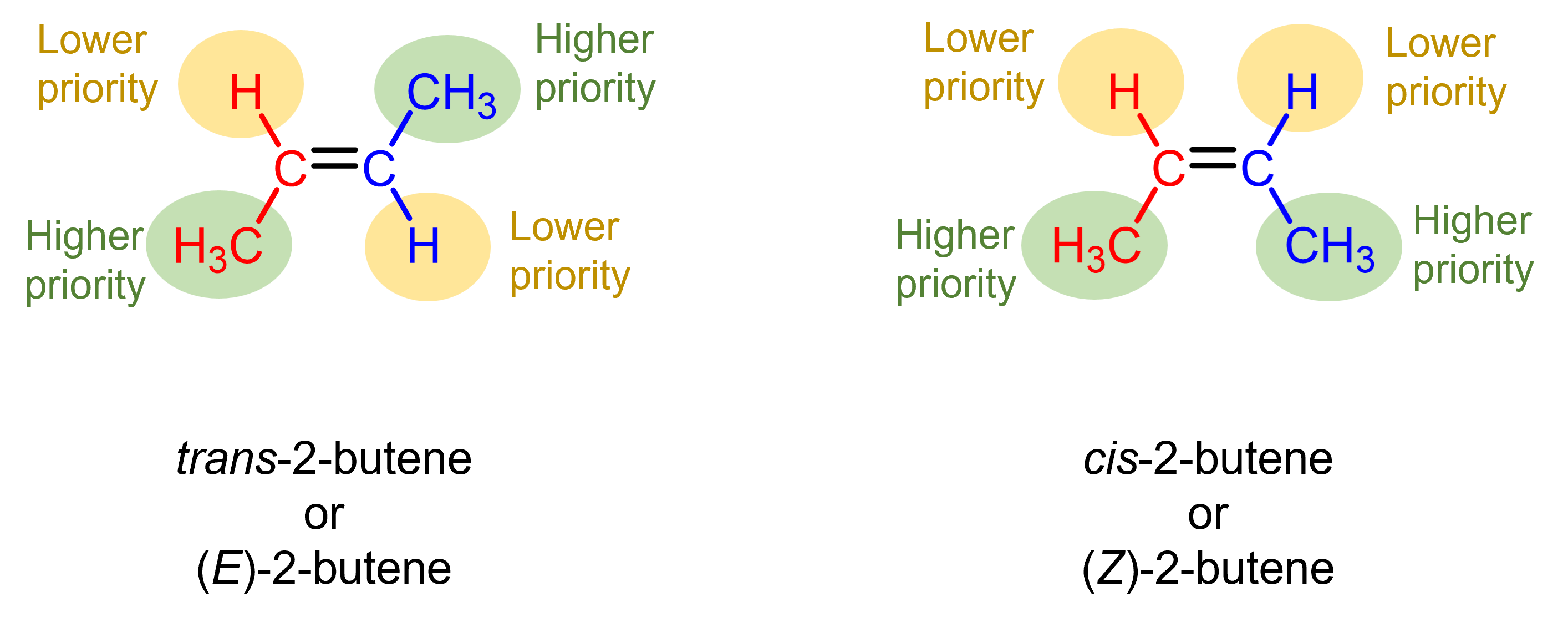
Similarly, we see that the higher priority groups (highlighted in green) are on the same side in cis-2-butene, and the nomenclature in CIP system for this molecule is (Z)-2-butene.
Let us consider a couple slightly more complex examples.
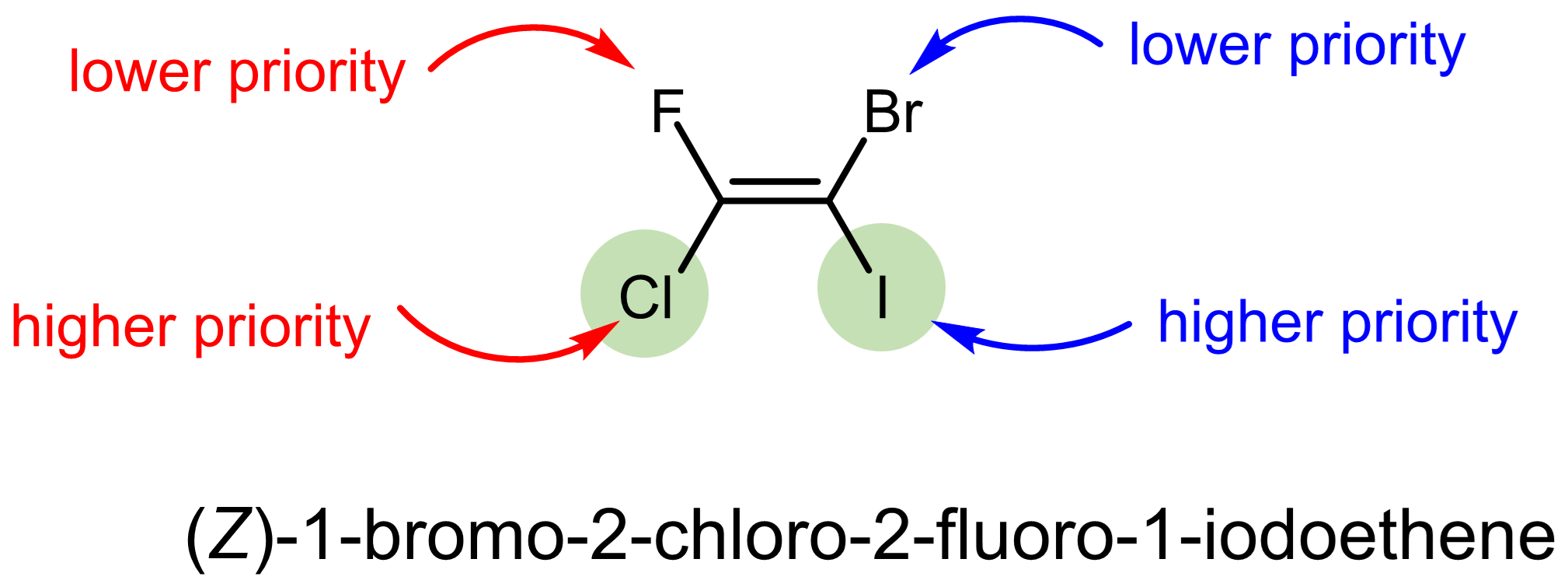
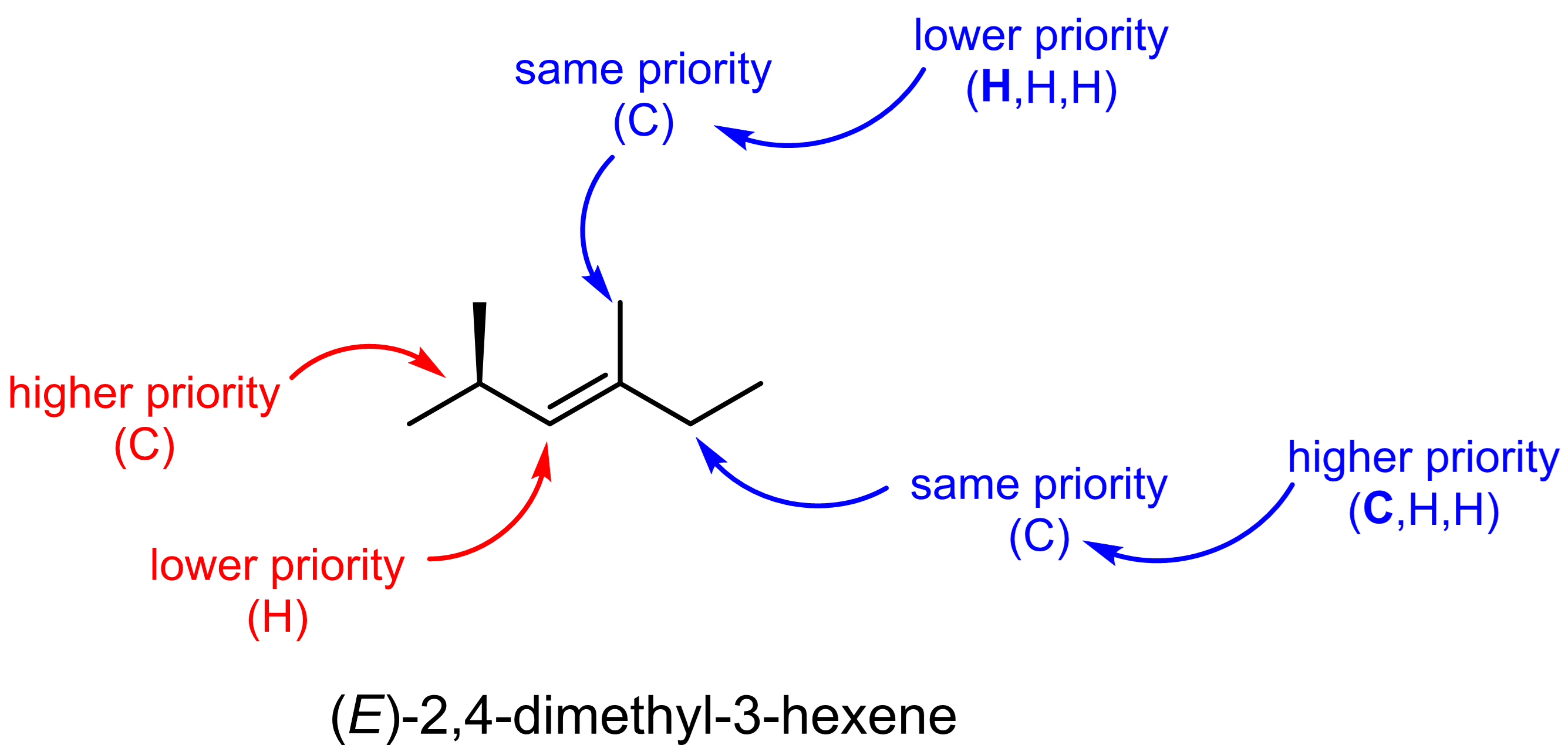
Exercise 4: CIP system
Label each of the stereocenters in the molecules below as (E) or (Z).
