17 Empirical Formula (M4Q4)
Introduction
Learning Objectives for Empirical Formulas
- Determine empirical and chemical formulas using composition by mass, combustion analysis data, and molar mass information.
| Determination of Empirical Formulas | Derivation of Molecular Formulas | Combustion Analysis |
| Key Concepts and Summary | Key Equations | Glossary | End of Section Exercises |
Determination of Empirical Formulas
As previously mentioned, the most common approach to determining a compound’s chemical formula is to first measure the masses of its constituent elements. However, we must keep in mind that chemical formulas represent the relative numbers, not masses, of atoms in the substance. Therefore, any experimentally derived data involving mass must be used to derive the corresponding numbers of atoms in the compound. To accomplish this, we can use molar masses to convert the mass of each element to a number of moles. We then consider the moles of each element relative to each other, converting these numbers into a whole-number ratio that can be used to derive the empirical formula of the substance. Consider a sample of compound determined to contain 1.71 g C and 0.287 g H. The corresponding numbers of atoms (in moles) are:
1.71 g C × [latex]\frac{1 \;\text{mol C}}{12.01 \;\text{g C}}[/latex] = 0.142 mol C
0.287 g H × [latex]\frac{1 \;\text{mol H}}{1.008 \;\text{g H}}[/latex] = 0.284 mol H
Thus, we can accurately represent this compound with the formula C0.142H0.248. Of course, per accepted convention, formulas contain whole-number subscripts, which can be achieved by dividing each subscript by the smaller subscript:
[latex]\text{C}_{\frac{0.142}{0.142}} \; \text{H}_{\frac{0.248}{0.142}}[/latex] or CH2
(Recall that subscripts of “1” are not written but rather assumed if no other number is present.)
The empirical formula for this compound is thus CH2. This may or not be the compound’s molecular formula as well; however, we would need additional information to make that determination (as discussed later in this section).
Consider as another example a sample of compound determined to contain 5.31 g Cl and 8.40 g O. Following the same approach yields a tentative empirical formula of:
Cl0.150O0.525 = [latex]\text{Cl}_{\frac{0.150}{0.150}} \; \text{O}_{\frac{0.525}{0.150}}[/latex] = ClO3.5
In this case, dividing by the smallest subscript still leaves us with a decimal subscript in the empirical formula. To convert this into a whole number, we must multiply each of the subscripts by two, retaining the same atom ratio and yielding Cl2O7 as the final empirical formula.
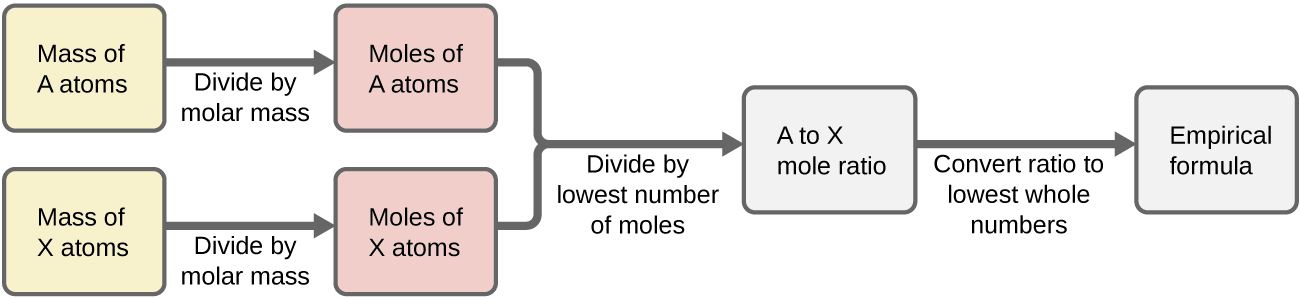
In summary, empirical formulas are derived from experimentally measured element masses by:
- Deriving the number of moles of each element from its mass
- Dividing each element’s molar amount by the smallest molar amount to yield subscripts for a tentative empirical formula
- Multiplying all coefficients by an integer, if necessary, to ensure that the smallest whole-number ratio of subscripts is obtained
Figure 1 outlines this procedure in flow chart fashion for a substance containing elements A and X.
Example 1
Determining a Compound’s Empirical Formula from the Masses of Its Elements
A sample of the black mineral hematite (Figure 2), an oxide of iron found in many iron ores, contains 34.97 g of iron and 15.03 g of oxygen. What is the empirical formula of hematite?

Solution
For this problem, we are given the mass in grams of each element. Begin by finding the moles of each:
34.97 g Fe × [latex]\frac{1\;\text{mol Fe}}{55.85\;\text{g Fe}}[/latex] = 0.6261 mol Fe
Next, derive the iron-to-oxygen molar ratio by dividing by the lesser number of moles:
[latex]\frac{0.6261}{0.6261}[/latex] = 1.000 mol Fe
[latex]\frac{0.9394}{0.6261}[/latex] = 1.500 mol O
The ratio is 1.000 mol of iron to 1.500 mol of oxygen (Fe1O1.5). Finally, multiply the ratio by two to get the smallest possible whole number subscripts while still maintaining the correct iron-to-oxygen ratio:
The empirical formula is Fe2O3.
Check Your Learning
What is the empirical formula of a compound if a sample contains 0.130 g of nitrogen and 0.370 g of oxygen?
Answer:
N2O5

For additional worked examples illustrating the derivation of empirical formulas, watch the brief video clip.
Deriving Empirical Formulas from Percent Composition
Finally, with regard to deriving empirical formulas, consider instances in which a compound’s percent composition is available rather than the absolute masses of the compound’s constituent elements. In such cases, the percent composition can be used to calculate the masses of elements present in any convenient mass of compound; these masses can then be used to derive the empirical formula in the usual fashion. Since the scale for percentages is 100, it is most convenient to assume a 100 g sample to calculate the mass of each element.
Example 2
Determining an Empirical Formula from Percent Composition
The bacterial fermentation of grain to produce ethanol forms a gas with a percent composition of 27.29% C and 72.71% O (Figure 3). What is the empirical formula for this gas?

Solution
Since the scale for percentages is 100, it is most convenient to calculate the mass of elements present in a sample weighing 100 g. The calculation is “most convenient” because, per the definition for percent composition, the mass of a given element in grams is numerically equivalent to the element’s mass percentage. This numerical equivalence results from the definition of the “percentage” unit, whose name is derived from the Latin phrase per centum meaning “by the hundred.” Considering this definition, the mass percentages provided may be more conveniently expressed as fractions:
27.29% C = [latex]\frac{27.29\;\text{g C}}{100\;\text{g compound}}[/latex]
72.71% O = [latex]\frac{72.71\;\text{g O}}{100\;\text{g compound}}[/latex]
The molar amounts of carbon and hydrogen in a 100-g sample are calculated by dividing each element’s mass by its molar mass:
27.29% C × [latex]\frac{\text{mol C}}{12.01\;\text{g}}[/latex] = 2.272 mol C
72.71% O × [latex]\frac{\text{mol O}}{16.00\;\text{g}}[/latex] = 4.544 mol O
Coefficients for the tentative empirical formula are derived by dividing each molar amount by the lesser of the two:
[latex]\frac{2.272\;\text{mol C}}{2.272\;\text{mol}}[/latex] = 1 C
[latex]\frac{4.544\;\text{mol O}}{2.272\;\text{mol}}[/latex] = 2 O
Since the resulting ratio is one carbon to two oxygen atoms, the empirical formula is CO2.
Check Your Learning
What is the empirical formula of a compound containing 40.0% C, 6.71% H, and 53.28% O?
Answer:
CH2O
Derivation of Molecular Formulas
Recall that empirical formulas are symbols representing the relative numbers of a compound’s elements. Determining the absolute numbers of atoms that compose a single molecule of a covalent compound requires knowledge of both its empirical formula and its molecular mass or molar mass. These quantities may be determined experimentally by various measurement techniques. Molecular mass, for example, is often derived from the mass spectrum of the compound (see discussion of this technique in the section on mass spectrometry of molecules from earlier in the semester). Molar mass can be measured by a number of experimental methods, many of which will be introduced in later chapters of this text.
Molecular formulas are derived by comparing the compound’s molecular or molar mass to its empirical formula mass. As the name suggests, an empirical formula mass is the sum of the average atomic masses of all the atoms represented in an empirical formula. If we know the molecular (or molar) mass of the substance, we can divide this by the empirical formula mass in order to identify the number of empirical formula units per molecule, which we designate as n:
[latex]\frac{\text{molecular or molar mass (amu or} \;\frac{\text{g}}{\text{mol}})}{\text{empirical formula mass (amu or} \;\frac{\text{g}}{\text{mol}})}[/latex] = n formula units/molecule
The molecular formula is then obtained by multiplying each subscript in the empirical formula by n, as shown by the generic empirical formula AxBy:
(AxBy)n = AnxBny
For example, consider a covalent compound whose empirical formula is determined to be CH2O. The empirical formula mass for this compound is approximately 30 amu (the sum of 12 amu for one C atom, 2 amu for two H atoms, and 16 amu for one O atom). If the compound’s molecular mass is determined to be 180 amu, this indicates that molecules of this compound contain six times the number of atoms represented in the empirical formula:
[latex]\frac{180 \;\text{amu/molecule}}{30\;\frac{\text{amu}}{\text{formula unit}}}[/latex] = 6 formula units/molecule
Molecules of this compound are then represented by molecular formulas whose subscripts are six times greater than those in the empirical formula:
(CH2O)6 = C6H12O6
Note that this same approach may be used when the molar mass (g/mol) instead of the molecular mass (amu) is used. In this case, we are merely considering one mole of empirical formula units and molecules, as opposed to single units and molecules.
Example 3
Determination of the Molecular Formula for Nicotine
Nicotine, an alkaloid in the nightshade family of plants that is mainly responsible for the addictive nature of cigarettes, contains 74.02% C, 8.710% H, and 17.27% N. If 40.57 g of nicotine contains 0.2500 mol nicotine, what is the molecular formula?
Solution
Determining the molecular formula from the provided data will require comparison of the compound’s empirical formula mass to its molar mass. As the first step, use the percent composition to derive the compound’s empirical formula. Assuming a convenient, a 100-g sample of nicotine yields the following molar amounts of its elements:
(74.02 g C)([latex]\frac{1\;\text{mol C}}{12.01\;\text{g C}}[/latex]) = 6.163 mol C
(8.710 g H)([latex]\frac{1\;\text{mol H}}{1.01\;\text{g H}}[/latex]) = 8.624 mol H
(17.27 g N)([latex]\frac{1\;\text{mol N}}{14.01\;\text{g N}}[/latex]) = 1.233 mol N
Next, we calculate the molar ratios of these elements relative to the least abundant element, N.
[latex]\frac{6.163\ \text{mol C}}{1.233\ \text{mol N}}[/latex] ≅ 5
[latex]\frac{8.264\ \text{mol H}}{1.233\ \text{mol N}}[/latex] ≅ 7
[latex]\frac{1.233\ \text{mol N}}{1.233\ \text{mol N}}[/latex] ≅ 1
The C-to-N and H-to-N molar ratios are adequately close to whole numbers, and so the empirical formula is C5H7N. The empirical formula mass for this compound is therefore 81.13 amu/formula unit, or 81.13 g/mol formula unit.
We calculate the molar mass for nicotine from the given mass and molar amount of compound:
Comparing the molar mass and empirical formula mass indicates that each nicotine molecule contains two formula units:
Thus, we can derive the molecular formula for nicotine from the empirical formula by multiplying each subscript by two:
Check Your Learning
What is the molecular formula of a compound with a percent composition of 49.47% C, 5.201% H, 28.84% N, and 16.48% O, and a molecular mass of 194.2 amu?
Answer:
C8H10N4O2
Combustion Analysis
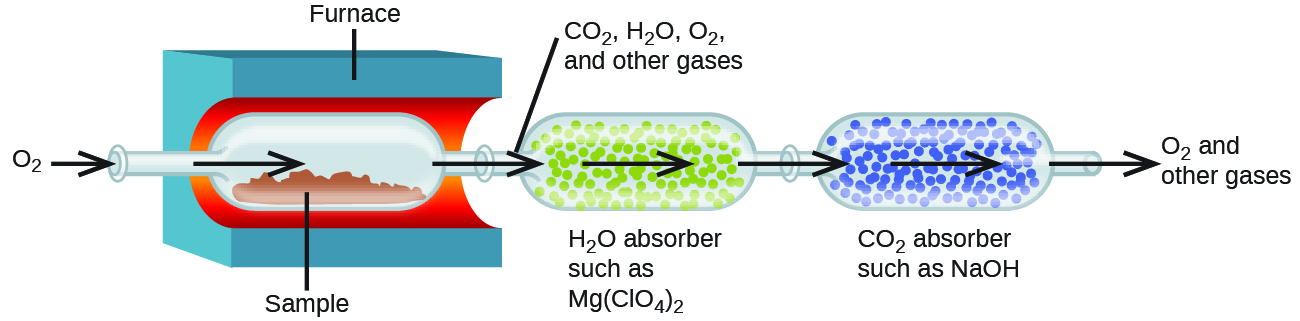
The elemental composition of hydrocarbons and related compounds may be determined via a method known as combustion analysis. In a combustion analysis, a weighed sample of the compound is heated to a high temperature under a stream of oxygen gas, resulting in its complete combustion to yield gaseous products of known identities. The complete combustion of hydrocarbons, for example, will yield carbon dioxide and water as the only products. The gaseous combustion products are swept through separate, preweighed collection devices containing compounds that selectively absorb each product (Figure 4). The mass increase of each device corresponds to the mass of the absorbed product and may be used in an appropriate stoichiometric calculation to derive the mass of the relevant element.
Example 4
Combustion Analysis
Polyethylene is a hydrocarbon (with formula CxHy) polymer used to produce food-storage bags and many other flexible plastic items. A combustion analysis of a 0.00126-g sample of polyethylene yields 0.00394 g of CO2 and 0.00161 g of H2O. What is the empirical formula of polyethylene?
Solution
The primary assumption in this exercise is that all the carbon in the sample combusted is converted to carbon dioxide, and all the hydrogen in the sample is converted to water:
Note that a balanced equation is not necessary for the task at hand. To derive the empirical formula of the compound, only the subscripts x and y are needed.
First, calculate the molar amounts of carbon and hydrogen in the sample, using the provided masses of the carbon dioxide and water, respectively. With these molar amounts, the empirical formula for the compound may be written as described earlier. An outline of this approach is given in the following flow chart:
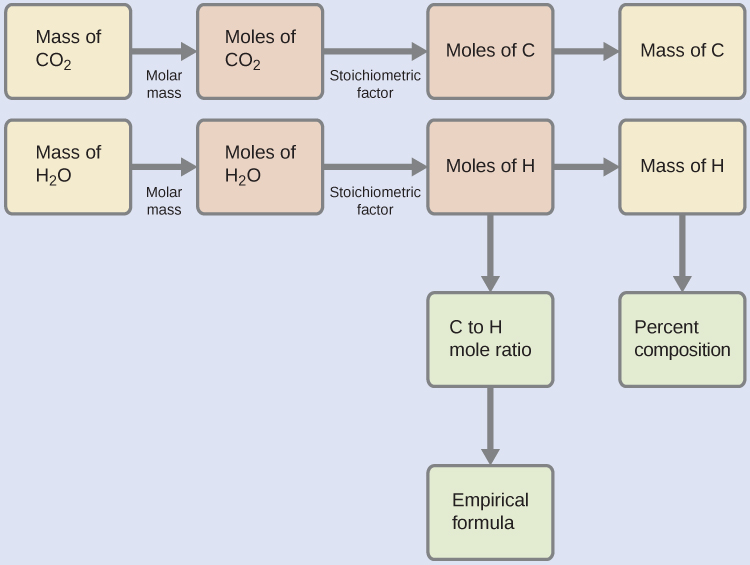
= 8.95 × 10-5 mol C
= 1.79 × 10-4 mol H
The empirical formula for the compound is then derived by identifying the smallest whole-number multiples for these molar amounts. The H-to-C molar ratio is
and the empirical formula for polyethylene is CH2.
Check Your Learning
A 0.00215-g sample of polystyrene, a polymer composed of carbon and hydrogen, produced 0.00726 g of CO2 and 0.00148 g of H2O in a combustion analysis. What is the empirical formula for polystyrene?
Answer:
CH
Example 5
Combustion Analysis of an Organic Compound Containing Oxygen
Combustion analysis of 23.1 mg of a white powder that contains only the elements C, H, and O yields 33.9 mg of CO2 and 13.9 mg of H2O. Determine the empirical formula of the white compound.
Solution
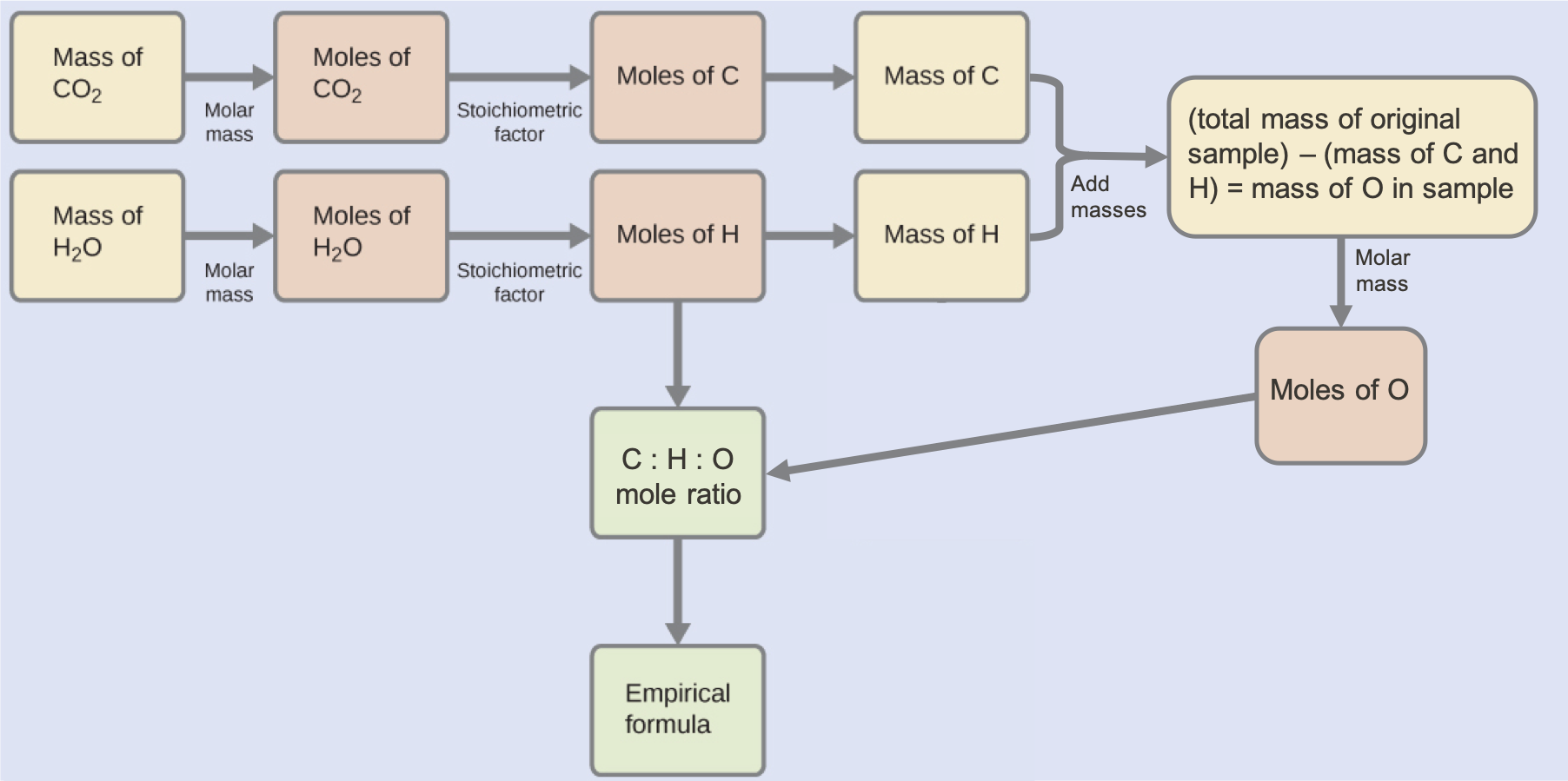
The mass of C and H in the white powder that produced the quantities of CO2 and H2O in the analysis must first be determined. This enables the amount of O in the compound to be calculated by subtraction, as this oxygen does not react in the combustion process.:
mass of C = 33.9 mg CO2 × [latex]\frac{1 \text{g}}{1000 \text{mg}}[/latex] × [latex]\frac{1 \text{mol CO}_2}{44.01 \text{g}}[/latex] × [latex]\frac{1 \text{mol C}}{1 \text{mol CO}_2}[/latex] × [latex]\frac{12.01 \text{g}}{1 \text{mol C}}[/latex] × [latex]\frac{1000 \text{mg}}{1 \text{g}}[/latex] = 9.25 mg C
mass of H = 13.9 mg H2O × [latex]\frac{1 \text{g}}{1000 \text{mg}}[/latex] × [latex]\frac{1 \text{mol H}_2 \text{O}}{18.02 \text{g}}[/latex] × [latex]\frac{2 \text{mol H}}{1 \text{mol H}_2 \text{O}}[/latex] × [latex]\frac{1.008 \text{g}}{1 \text{mol H}}[/latex] × [latex]\frac{1000 \text{mg}}{1 \text{g}}[/latex] = 1.54 mg H
Because the white powder consists of just three elements and we now know the mass of two of them, the mass of O is the remainder:
mass O = 23.1 mg – (9.25 mg + 1.54 mg) = 12.3 mg O
Now the moles of each element can be determined, and the empirical formula determined:
moles C: 9.25 mg C × [latex]\frac{1\;\text{g}}{1000\;\text{mg}}[/latex] × [latex]\frac{1\;\text{mol C}}{12.01\;\text{g}}[/latex] = 7.70 × 10-4 mol C
moles H: 1.54 mg H × [latex]\frac{1\;\text{g}}{1000\;\text{mg}}[/latex] × [latex]\frac{1\;\text{mol H}}{1.008\;\text{g}}[/latex] = 1.53 × 10-3 mol H
moles O: 12.3 mg O × [latex]\frac{1\;\text{g}}{1000\;\text{mg}}[/latex] × [latex]\frac{1\;\text{mol C}}{16.00\;\text{g}}[/latex] = 7.69 × 10-4 mol O
Dividing each of these by the smallest number of moles (7.69 x 10-4 mol) yields the empirical formula: CH2O
This approach is outlined in the following flow chart:
Check Your Learning
Combustion analysis of 0.112 g of a compound that contains C, H, and O yields 0.0561 g of H2O and 0.205 g of CO2. The hard mass spectrum shows three peaks at 1, 12, and 16 amu, and the soft mass spectrum a single peak at 144 amu. Determine the empirical and actual formula of the compound.
Answer:
The hard mass spectrum indicates the compound consists of the elements C, H, and O.
Empirical Formula C3H4O2, actual Formula C6H8O4
Key Concepts and Summary
The chemical identity of a substance is defined by the types and relative numbers of atoms composing its fundamental entities. To determine an empirical formula, you must find the smallest whole-number ratio of atoms in a molecule. If you begin with the mass of each element in the compound, you must first convert these masses to moles before determining the ratio. A compound’s percent composition provides the mass percentage of each element in the compound, and it is often experimentally determined and used to derive the compound’s empirical formula. The empirical formula mass of a covalent compound may be compared to the compound’s molecular or molar mass to derive a molecular formula. Combustion analysis can be used to identify an unknown hydrocarbon because the identity of the products are known to be CO2 and H2O.
Key Equations
- %X = [latex]\frac{\text{mass X}}{\text{mass compound}}[/latex] × 100%
- [latex]\frac{\text{molecular or molar mass (amu or} \;\frac{\text{g}}{\text{mol}})}{\text{empirical formula mass (amu or} \;\frac{\text{g}}{\text{mol}})}[/latex] = n formula units/molecule
- (AxBy)n = AnxBny
Glossary
- empirical formula mass
- sum of average atomic masses for all atoms represented in an empirical formula
-
- combustion analysis
- analyical technique used to determine the elemental composition of a compound via the collection and weighing of its gaseous combustion products
- percent composition
- percentage by mass of the various elements in a compound
Chemistry End of Section Exercises
Empirical Formula
- What information do we need to determine the molecular formula of a compound from the empirical formula?
- Calculate the following to four significant figures:
- the percent composition of ammonia, NH3
- the percent composition of photographic “hypo,” Na2S2O3
- the percent of calcium ion in Ca3(PO4)2
- Determine the percent ammonia, NH3, in Co(NH3)6Cl3, to three significant figures.
- Determine the empirical formulas for compounds with the following percent compositions:
- 15.8% carbon and 84.2% sulfur
- 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen
- A compound contains carbon, hydrogen, and chlorine. It has a molar mass of 99 g/mol. Analysis of a sample shows that it contains 24.3% carbon and 4.1% hydrogen. What is its molecular formula?
- Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol.
- Polymers are large molecules composed of simple units repeated many times. Thus, they often have relatively simple empirical formulas. Calculate the empirical formulas of the following polymers:
- Lucite (Plexiglas); 59.9% C, 8.06% H, 32.0% O
- Saran; 24.8% C, 2.0% H, 73.1% Cl
- polyethylene; 86% C, 14% H
- polystyrene; 92.3% C, 7.7% H
- Orlon; 67.9% C, 5.70% H, 26.4% N
- A major textile dye manufacturer developed a new yellow dye. The dye has a percent composition of 75.95% C, 17.72% N, and 6.33% H by mass with a molar mass of about 240 g/mol. Determine the molecular formula of the dye.
Combustion Analysis
- A 0.025-g sample of a compound composed of boron and hydrogen, with a molecular mass of ~28 amu, burns spontaneously when exposed to air, producing 0.063 g of B2O3. What are the empirical and molecular formulas of the compound? The general reaction is
BxHy + O2 → B2O3 + H2O (not balanced)
- A compound has a molecular mass of about 130 amu, and contains only carbon and hydrogen. A 3.000-mg sample of this compound burns to give 10.3 mg of CO2. Determine its empirical and molecular formulas.
- Combustion analysis of 0.112 g of a compound that contains C, H, and O yields 0.0561 g of H2O and 0.205 g of CO2. The hard mass spectrum shows three peaks at 1, 12, and 16 amu, and the soft mass spectrum shows a single peak at 144 amu. Determine the empirical and actual formula of the compound.
Answers to Chemistry End of Section Exercises
- You need a given mass and the molar amount of the compound to get the molar mass so it can be compared to the empirical mass
- (a) % N = 82.24%, % H = 17.76%; (b) % Na = 29.08%, % S = 40.56%, % O = 30.36%; (c) % Ca2+ = 38.76%
- % NH3 = 38.2%
- (a) CS2; (b) CH2O
- C2H4Cl2
- Mg3Si2H3O8 (empirical formula), Mg6Si4H6O16 (molecular formula)
- (a) C5H8O2; (b) CHCl; (c) CH2; (d) CH; (e) C3H3N
- C15H15N3
- The empirical formula is BH3. The molecular formula is B2H6.
- Empirical formula: C5H4, molecular formula: C10H8
- The hard mass spectrum indicates the compound consists of the elements C, H, and O. Empirical formula: C3H4O2, molecular formula: C6H8O4

