54 Melting and Boiling Point Comparisons (M10Q2)
Introduction
Learning Objectives for Melting and Boiling Point Comparisons
- Connect trends in physical properties with relative strength of intermolecular forces.
- Predict relative melting or boiling points of similar compounds.
| Melting Point and Boiling Point Comparisons | Effect of Hydrogen Bonding on Boiling Point |
- Predict relative melting or boiling points of similar compounds.
Melting Point and Boiling Point Comparisons
In the previous section, we compared F2 and Cl2 (gases at room temperature) to Br2 (a liquid at room temperature), and I2 (a solid at room temperature). All of these molecules have London forces as their primary IMF since they are nonpolar molecules. We determined that I2 must have the strongest IMFs due to existing in the solid state and that F2 and Cl2 must have the weakest IMFs. But how might we compare the IMFs between F2 and Cl2, two gases at the same temperature and pressure? To do this, we can make use of melting point and boiling point data, as seen in Table 1. A higher melting and boiling point indicates that more kinetic energy is required to overcome the IMFs and melt or boil the substance. Examining the melting and boiling points for these halogens shows a trend that larger and heavier atoms display stronger IMFs, as shown by the higher melting and boiling points.
| Halogen | Molar Mass | Atomic Radius | Melting Point | Boiling Point |
|---|---|---|---|---|
| fluorine, F2 | 38 g/mol | 72 pm | 53 K | 85 K |
| chlorine, Cl2 | 71 g/mol | 99 pm | 172 K | 238 K |
| bromine, Br2 | 160 g/mol | 114 pm | 266 K | 332 K |
| iodine, I2 | 254 g/mol | 133 pm | 387 K | 457 K |
| astatine, At2 | 420 g/mol | 150 pm | 575 K | 610 K |
| Table 1. Melting and Boiling Points of the Halogens | ||||
The increase in melting and boiling points with increasing atomic/molecular size may be rationalized by considering how the strength of dispersion forces is affected by the electronic structure of the atoms or molecules in the substance.
Example 1
London Forces and Their Effects
Order the following compounds of a group 14 element and hydrogen from lowest to highest boiling point: CH4, SiH4, GeH4, and SnH4. Explain your reasoning.
Solution
Applying the skills acquired in the module on chemical bonding and molecular geometry, all of these compounds are predicted to be nonpolar, so they may experience only dispersion forces: the smaller the molecule, the less polarizable and the weaker the dispersion forces; the larger the molecule, the larger the dispersion forces. The molar masses of CH4, SiH4, GeH4, and SnH4 are approximately 16 g/mol, 32 g/mol, 77 g/mol, and 123 g/mol, respectively. Therefore, CH4 is expected to have the lowest boiling point and SnH4 the highest boiling point. The ordering from lowest to highest boiling point is expected to be CH4 < SiH4 < GeH4 < SnH4.
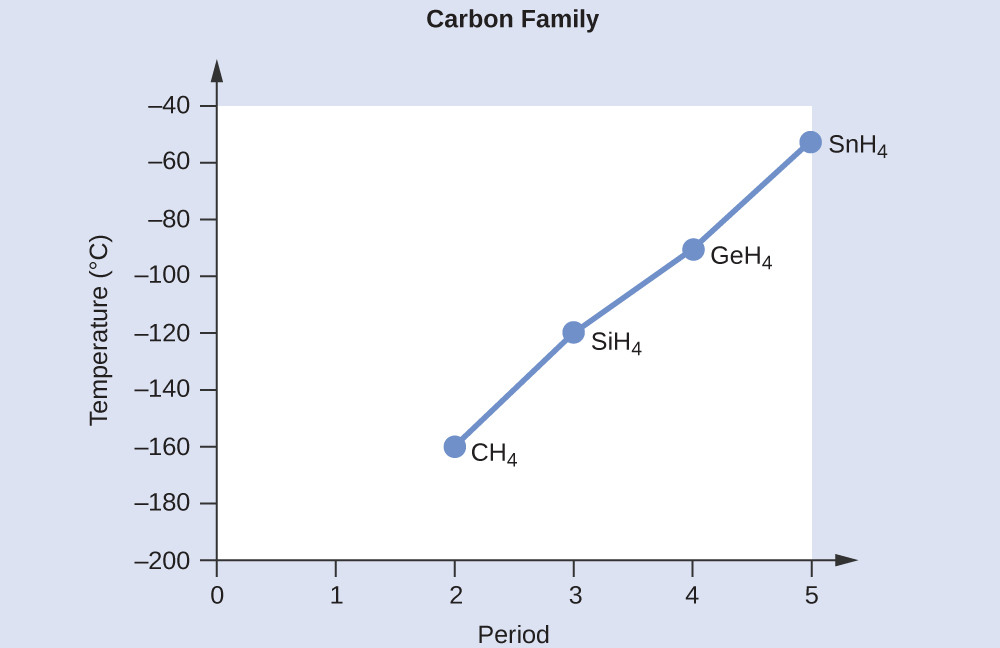
A graph of the actual boiling points of these compounds versus the period of the group 14 element shows this prediction to be correct:
Check Your Learning
Order the following hydrocarbons from lowest to highest boiling point: C2H6, C3H8, and C4H10.
Answer:
C2H6 < C3H8 < C4H10. All of these compounds are nonpolar and only have London dispersion forces: the larger the molecule, the larger the dispersion forces and the higher the boiling point. The ordering from lowest to highest boiling point is therefore C2H6 < C3H8 < C4H10.
Example 2
Effect of Shape on London Forces and Boiling Points
As seen in the previous module, the shapes of molecules also affect the magnitudes of the dispersion forces between them. The previous section compared the isomers isopentane, n-pentane, and neopentane and ranked the strength of their IMFs. The boiling points of these isomers are 9.5 °C, 27 °C and 36 °C, in no particular order. Using what you know about how London forces are affected by the shape of a molecule, determine which boiling point corresponds to which isomer. Explain how you came to your conclusions. It may be useful to revisit the structures.
Solution
We saw in the previous section that n-pentane has the most elongated structure and that this increased surface area available for contact between molecules resulted in a stronger London force. Therefore, we would predict that n-pentane has the highest boiling point of 36 °C due to its strong dispersion forces. Since neopentane molecules are the most compact of the three and have the least available surface area for intermolecular contact, we would predict that this molecule would have a boiling point of 9.5 °C. This means that isopentane, having an intermediate surface area, would have a boiling point of 27 °C.
Now let’s compare two molecules with the same number of atoms and similar molecular weight, HCl and F2. Both of these molecules experience London forces, but only HCl has a dipole moment and would therefore experience dipole-dipole interactions. The higher normal boiling point (the boiling point at 1 atm, as will be discussed in the next section) of HCl (188 K) compared to F2 (85 K) is a reflection of the greater strength of dipole-dipole attractions between HCl molecules, compared to the attractions between nonpolar F2 molecules. We will often use values such as boiling or freezing points, or enthalpies of vaporization or fusion, as indicators of the relative strengths of IMFs of attraction present within different substances.
Dipole-dipole interactions do not definitively indicate a higher boiling point, however. Consider the trends in boiling points for the binary hydrides of group 15 (NH3, PH3, AsH3, and SbH3), group 16 hydrides (H2O, H2S, H2Se, and H2Te), and group 17 hydrides (HF, HCl, HBr, and HI). The boiling points of the heaviest three hydrides for each group are plotted in Figure 1. As we progress down any of these groups, the polarities of the molecules decrease slightly, whereas the sizes of the molecules increase substantially. The effect of increasingly stronger dispersion forces dominates that of increasingly weaker dipole-dipole attractions, and the boiling points are observed to increase steadily.
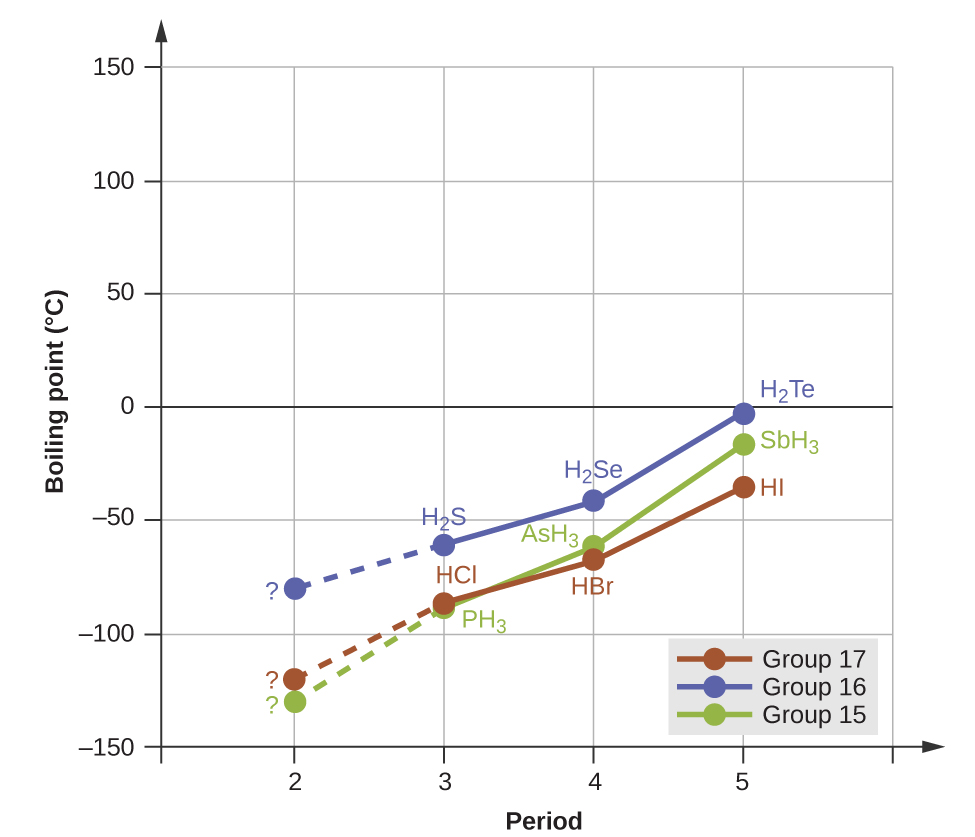
Example 3
Dipole-Dipole Forces and Their Effects
Predict which will have the higher boiling point: N2 or CO. Explain your reasoning.
Solution
CO and N2 are both diatomic molecules with masses of about 28 amu, so they experience similar London dispersion forces. Because CO is a polar molecule, it experiences dipole-dipole attractions. Because N2 is nonpolar, its molecules cannot exhibit dipole-dipole attractions. The dipole-dipole attractions between CO molecules are comparably stronger than the dispersion forces between nonpolar N2 molecules, so CO is expected to have the higher boiling point.
Check Your Learning
Predict which will have the higher boiling point: ICl or Br2. Explain your reasoning.
Answer:
ICl. ICl and Br2 have similar masses (~160 amu) and therefore experience similar London dispersion forces. ICl is polar and thus also exhibits dipole-dipole attractions; Br2 is nonpolar and does not. The relatively stronger dipole-dipole attractions require more energy to overcome, so ICl will have the higher boiling point.
The Effect of Hydrogen Bonding on Boiling Point
The previous section introduced hydrogen bonds as a unique type of dipole-dipole interaction that occurs between an H atom attached to an N, O, or F atom on one molecule and the lone pairs on another N, O, or F atom on another molecule. If we use the trend in Figure 1 above to predict the boiling points for the lightest hydride for each group, we would expect NH3 to boil at about −120 °C, H2O to boil at about −80 °C, and HF to boil at about −110 °C. However, when we measure the boiling points for these compounds, we find that they are dramatically higher than the trends would predict, as shown in Figure 2. The stark contrast between our naïve predictions and reality provides compelling evidence for the strength of hydrogen bonding.
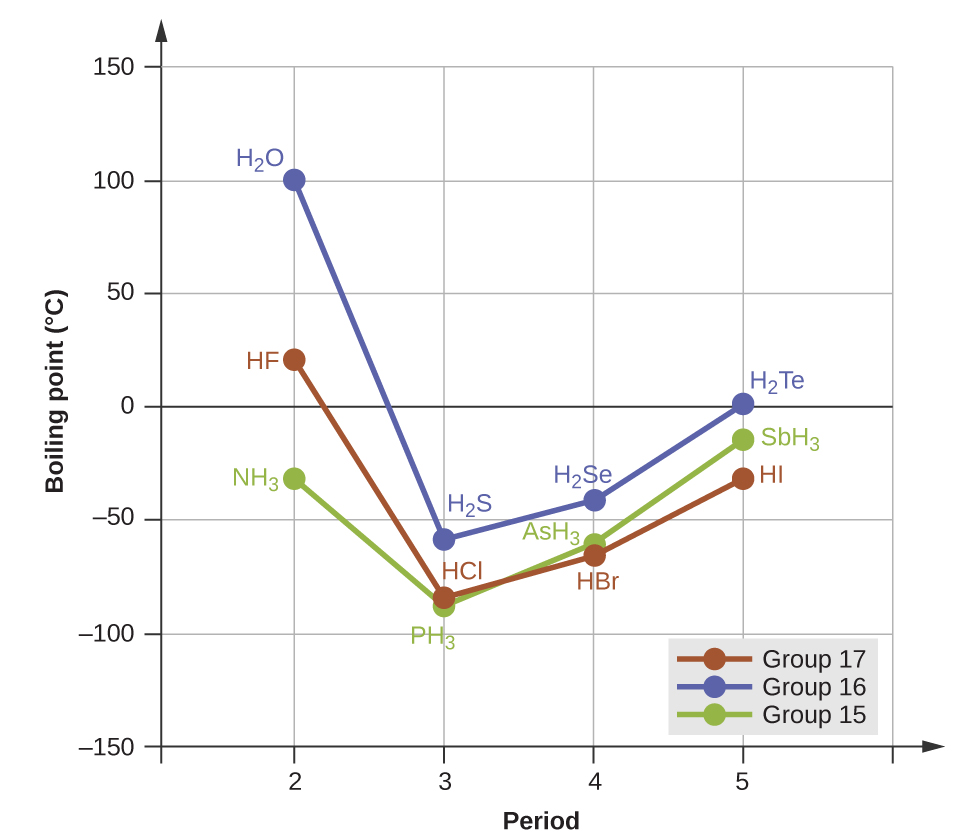
Example 4
Effect of Hydrogen Bonding on Boiling Points
Consider the compounds dimethylether (CH3OCH3), ethanol (CH3CH2OH), and propane (CH3CH2CH3). Their boiling points, not necessarily in order, are −42.1 °C, −24.8 °C, and 78.4 °C. Match each compound with its boiling point. Explain your reasoning.
Solution
The VSEPR-predicted shapes of CH3OCH3, CH3CH2OH, and CH3CH2CH3 are similar, as are their molar masses (46 g/mol, 46 g/mol, and 44 g/mol, respectively), so they will exhibit similar dispersion forces. Since CH3CH2CH3 is nonpolar, it will exhibit only London dispersion forces. Because CH3OCH3 is polar, it will also experience dipole-dipole attractions. Finally, CH3CH2OH has an −OH group, and so it will experience the uniquely strong dipole-dipole attraction known as hydrogen bonding. So the ordering in terms of strength of IMFs, and thus boiling points, is CH3CH2CH3 < CH3OCH3 < CH3CH2OH. The boiling point of propane is −42.1 °C, the boiling point of dimethylether is −24.8 °C, and the boiling point of ethanol is 78.5 °C.
Check Your Learning
Ethane (CH3CH3) has a melting point of −183 °C and a boiling point of −89 °C. Predict the melting and boiling points for methylamine (CH3NH2). Explain your reasoning.
Answer:
The melting point and boiling point for methylamine are predicted to be significantly greater than those of ethane. CH3CH3 and CH3NH2 are similar in size and mass, but methylamine possesses an −NH group and therefore may exhibit hydrogen bonding. This greatly increases its IMFs, and therefore its melting and boiling points. It is difficult to predict values, but the known values are a melting point of −93 °C and a boiling point of −6 °C.
Key Concepts and Summary
The physical properties of a substance depend on the IMFs that the substance experiences. The first step in predicting these physical properties is determining the type of IMFs that are present, which we discussed in the previous section. Some of these IMFs, such as dipole-dipole attraction, depend on the polarity of the molecule. The stronger the IMFs, the more energy that will be needed to melt or boil the substance. This means that the higher the melting or boiling point of a substance, the stronger its IMFs must be.
Chemistry End of Section Exercises
- Why do the boiling points of the noble gases increase in the order He < Ne < Ar < Kr < Xe?
- Neon and HF have approximately the same molecular masses.
- Explain why the boiling points of Neon and HF differ.
- Compare the change in the boiling points of Ne, Ar, Kr, and Xe with the change of the boiling points of HF, HCl, HBr, and HI, and explain the difference between the changes with increasing atomic or molecular mass.
- Arrange each of the following sets of compounds in order of increasing boiling point temperature:
- HCl, H2O, SiH4
- F2, Cl2, Br2
- CH4, C2H6, C3H8
- O2, NO, N2
- The molecular mass of butanol, C4H9OH, is 74.14; that of ethylene glycol, CH2(OH)CH2OH, is 62.08, yet their boiling points are 117.2 °C and 174 °C, respectively. Explain the reason for the difference.
- On the basis of intermolecular attractions, explain the differences in the boiling points of n–butane (−1 °C) and chloroethane (12 °C), which have similar molar masses.
- On the basis of dipole moments and/or hydrogen bonding, explain in a qualitative way the differences in the boiling points of acetone (56.2 °C) and 1-propanol (97.4 °C), which have similar molar masses.
- The melting point of H2O(s) is 0 °C. Would you expect the melting point of H2S(s) to be −85 °C, 0 °C, or 185 °C? Explain your answer.
- Silane (SiH4), phosphine (PH3), and hydrogen sulfide (H2S) melt at −185 °C, −133 °C, and −85 °C, respectively. What does this suggest about the polar character and intermolecular attractions of the three compounds?
Answers to Chemistry End of Section Exercises
- The London dispersion forces typically increase as the number of electrons increase.
- (a) In additional to London dispersion forces, HF also has hydrogen bonding, therefore it has higher boiling point than neon.
(b) Ne < Ar < Kr < Xe: boiling point increases with increasing atomic mass and dispersion forces. HCl< HBr < HI < HF: HF has the highest boiling point because of additional hydrogen bonding, even though it has the lowest molecular mass. - (a) SiH4 < HCl < H2O; (b) F2 < Cl2 < Br2; (c) CH4 < C2H6 < C3H8; (d) N2 < O2 < NO
- The presence of two -OH groups in ethylene glycol leads to twice as many hydrogen-bond formation between ethylene glycol molecules compared to butanol, which has one -OH group. This leads to increase in boiling point because more energy is needed to overcome the stronger IMFs.
- Only London dispersion forces are available to hold n-butane in the liquid state. Chloroethane, however, has rather large dipole interactions; the overall IMFs is therefore stronger, leading to a higher boiling point.
- They have similar London dispersion forces and dipole-dipole attractions. But 1-propanol contains an OH group, which allows for hydrogen bonding. Therefore 1-propanol has higher boiling point.
- −85 °C. Water has stronger hydrogen bonds so it melts at a higher temperature.
- The higher the melting point of a substance, the stronger its IMFs must be. SiH4 has the weakest intermolecular forces and it is non-polar, H2S has the strongest intermolecular forces and it is the most polar molecule here.

