2 States of Matter (M1Q2)
Introduction
Learning Objectives for States of Matter
- Identify and describe the basic properties of each physical state of matter: solid, liquid, and gas.
| Key Concepts and Summary | Glossary | End of Section Exercises |
Matter
Matter on the Macroscopic Scale
Matter is defined as anything that occupies space and has mass, and it is all around us. Solids and liquids are more obviously matter: we can see that they take up space, and their weight tells us that they have mass. Gases are also matter; if gases did not take up space, a balloon would stay collapsed rather than inflate when filled with gas.
Solids, liquids, and gases are the three states of matter commonly found on earth (Figure 1). A solid is rigid and possesses a definite shape. A liquid flows and takes the shape of a container, except that it forms a flat or slightly curved upper surface when acted upon by gravity. (In zero gravity, liquids assume a spherical shape.) Both liquid and solid samples have volumes that are very nearly independent of pressure. A gas takes both the shape and volume of its container.
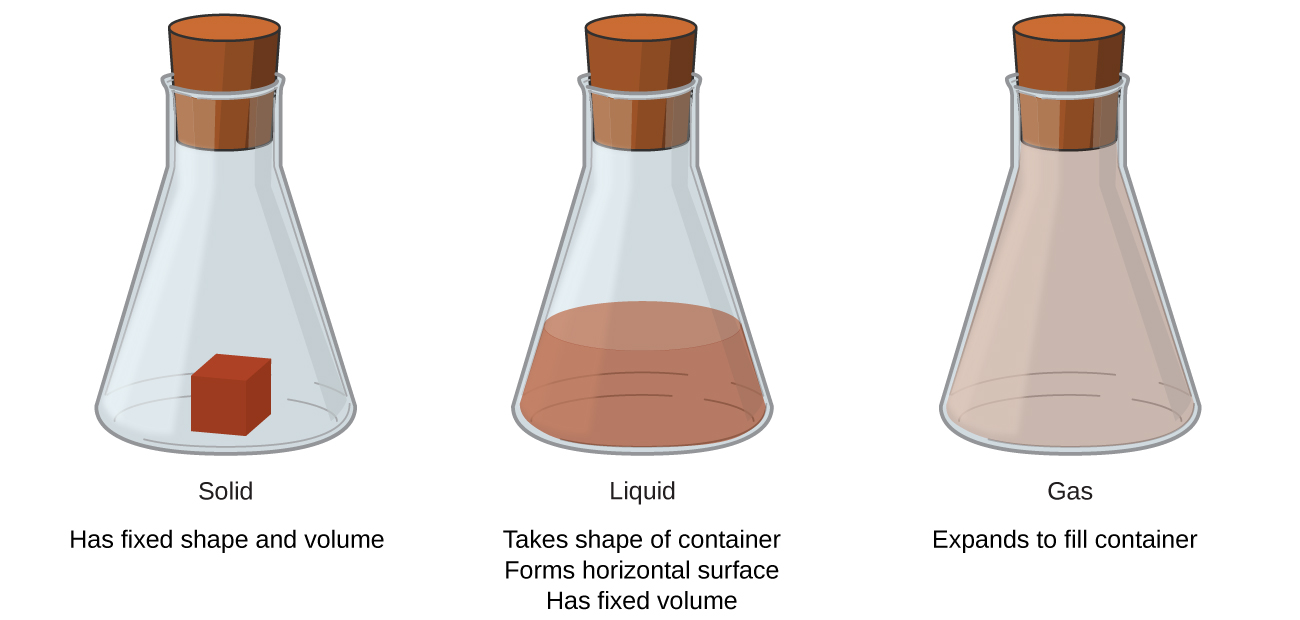
A fourth state of matter, plasma, occurs naturally in the interiors of stars. A plasma is a gaseous state of matter that contains appreciable numbers of electrically charged particles (Figure 2). The presence of these charged particles imparts unique properties to plasmas that justify their classification as a state of matter distinct from gases. In addition to stars, plasmas are found in some other high-temperature environments (both natural and man-made), such as lightning strikes, certain television screens, and specialized analytical instruments used to detect trace amounts of metals.


In a tiny cell in a plasma television, the plasma emits ultraviolet light, which in turn causes the display at that location to appear a specific color. The composite of these tiny dots of color makes up the image that you see. Watch this video to learn more about plasma and the places you encounter it.
Some samples of matter appear to have properties of solids, liquids, and/or gases at the same time. This can occur when the sample is composed of many small pieces. For example, we can pour sand as if it were a liquid because it is composed of many small grains of solid sand. Matter can also have properties of more than one state when it is a mixture, such as with clouds. Clouds appear to behave somewhat like gases, but they are actually mixtures of air (gas) and tiny particles of water (liquid or solid).
Matter on the Sub-Microscopic Scale
We can observe distinct properties of solids, liquids, and gases at the macroscopic scale, but it is difficult to directly observe matter at the particle level. We can generate models and simulations, such as the States of Matter PhET simulation, to help visualize how the particles of solids, liquids, and gases behave differently. It is these differences of particle behavior that lead to the observable properties on the macroscopic-level. As a brief introduction, we will examine how neon particles behave in the different states (Figure 3). When neon is below its melting point (24.56 K), then it is in the solid state. The particles rigidly interact, the shape and volume are fixed, and the particles paper writing help vibrate. When neon is between its melting and boiling points, then it is in the liquid state. The particles still interact and the volume is fixed. However, liquid particles can slide past one and another, and it takes on the shape of the container. Above its boiling point (27.10 K), then it is in the gaseous state. The particles interact very little, and they are moving very rapidly past each other. The gas particles expand to take on the shape and volume of the container.
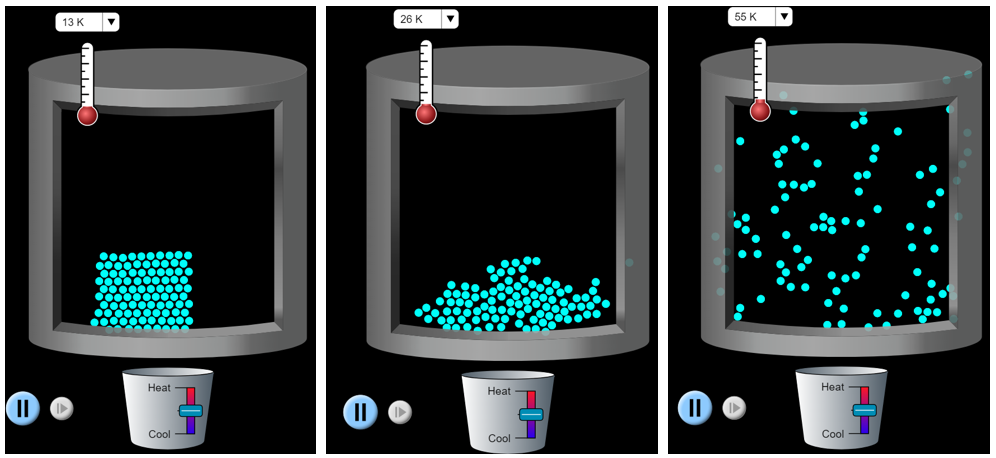
Different types of matter have different melting and boiling points. For example, water melts at 0 °C (273.15 K) and boils at 100 °C (373.15 K). We will go into greater depth about how particles interact during the rest of this course. For now, it is acceptable to think of water particles as being “stickier” than neon particles. Thus, more energy must be applied (and a higher temperature reached) before water transitions from the solid to liquid states.
Key Concepts and Summary
Matter is anything that occupies space and has mass. On earth, matter commonly exists in three states: solids, of fixed shape and volume; liquids, of variable shape but fixed volume; and gases, of variable shape and volume. Under high-temperature conditions, matter also can exist as a plasma.
Glossary
-
- gas
- state in which matter has neither definite volume nor shape
- liquid
- state of matter that has a definite volume but indefinite shape
-
- matter
- anything that occupies space and has mass
- plasma
- gaseous state of matter containing a large number of electrically charged atoms and/or molecules
- solid
- state of matter that is rigid, has a definite shape, and has a fairly constant volume
Chemistry End of Section Exercises
- What properties distinguish solids from liquids? Liquids from gases? Solids from gases?
- How does your answer to question #1 vary depending on if you are discussing the macroscopic or sub-microscopic level?
- The halogens fluorine, iodine, and bromine are each a different state at room temperature: fluorine is a gas, iodine is a solid, and bromine is a liquid. Rank the halogen particles from most “sticky” to least “sticky”.
Answers to Chemistry End of Section Exercises
- Liquids can change their shape (flow); solids can’t. Gases can undergo large volume changes as pressure changes; liquids do not. Gases flow and change volume; solids do not.
- At the sub-microscopic level, the differences arise from how particles move. For solids, the particles are rigid and therefore do not move (only vibrate), giving rise to the fixed shape at the macroscopic level. For liquids, the particles are less rigid in the sense they slide past each other, which explains how they have a fixed volume at the macroscopic level but still fill the shape of the container. And for gases, the particles become very spread out and can move freely about the container.
- Most “sticky” particles: iodine [solid at room temperature]; bromine [liquid at room temperature]; least “sticky” particles: fluorine [gas at room temperature]

