59 Types of Solids (M11Q3)
Introduction
We now begin to look more closely at the solid phase of matter. In this section we will see that solids can have different structures and bonding motifs. These characteristics will then affect the physical properties of the solid.
Learning Objectives for Types of Solids and Unit Cell Definitions
- Distinguish between various structures (amorphous or crystalline), bonding motifs (metallic, covalent network, ionic, or molecular) of solids, and the resulting physical properties (e.g. melting point, ductility).
| Crystalline & Amorphous Solids | Metallic Solids | Covalent Network Solids | Ionic Solids | Molecular Solids | Properties of Solids |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Crystalline & Amorphous Solids
When most liquids are cooled, they eventually freeze and form crystalline solids, solids in which the atoms, ions, or molecules are arranged in a definite repeating pattern. It is also possible for a liquid to freeze before its molecules become arranged in an orderly pattern. The resulting materials are called amorphous solids or noncrystalline solids (or, sometimes, glasses). The particles of such solids lack an ordered internal structure and are randomly arranged (Figure 1).
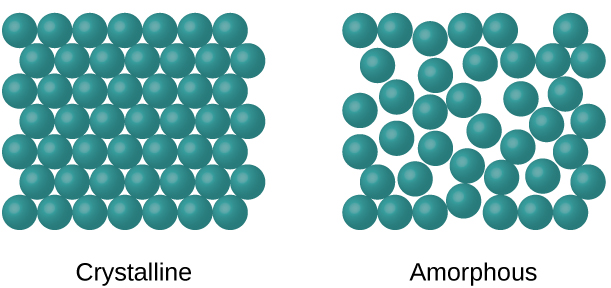
Metals and ionic compounds typically form ordered, crystalline solids. Substances that consist of large molecules, or a mixture of molecules whose movements are more restricted, often form amorphous solids. For example, candle waxes are amorphous solids composed of large hydrocarbon molecules. Some substances, such as boron oxide (shown in Figure 2), can form either crystalline or amorphous solids, depending on the conditions under which it is produced. Also, amorphous solids may undergo a transition to the crystalline state under appropriate conditions.
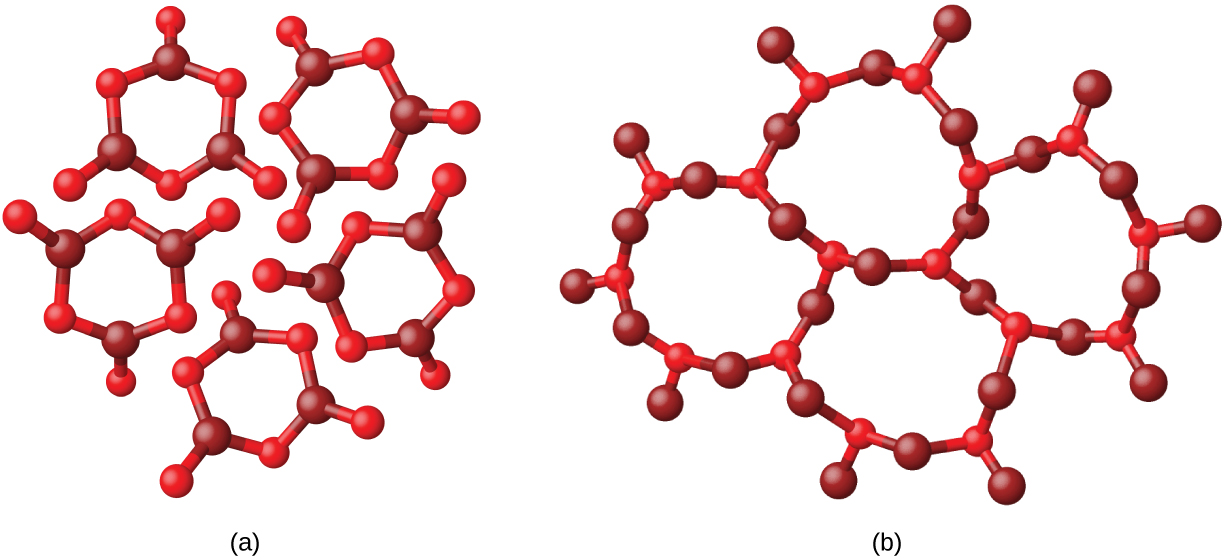
Crystalline solids are generally classified according to the nature of the forces that hold its particles together. These forces are primarily responsible for the physical properties exhibited by the bulk solids. The following sections provide descriptions of the major types of crystalline solids: metallic, covalent network, ionic, and molecular.
Metallic Solids
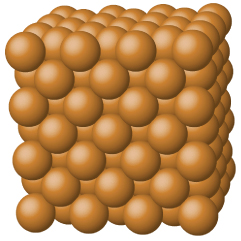
Metallic solids such as crystals of copper, aluminum, and iron are formed by metal atoms (Figure 3). The structure of metallic crystals is often described as a uniform distribution of atomic nuclei within a “sea” of delocalized electrons. The atoms within such a metallic solid are held together by a unique force known as metallic bonding that gives rise to many useful and varied bulk properties. All exhibit high thermal and electrical conductivity, metallic luster, and malleability. Many are very hard and quite strong. Because of their malleability (the ability to deform under pressure or hammering), they do not shatter and, therefore, make useful construction materials. The melting points of the metals vary widely. Mercury is a liquid at room temperature, and the alkali metals melt below 200 °C. Several post-transition metals also have low melting points, whereas the transition metals melt at temperatures above 1000 °C. These differences reflect differences in strengths of metallic bonding among the metals.
Covalent Network Solids
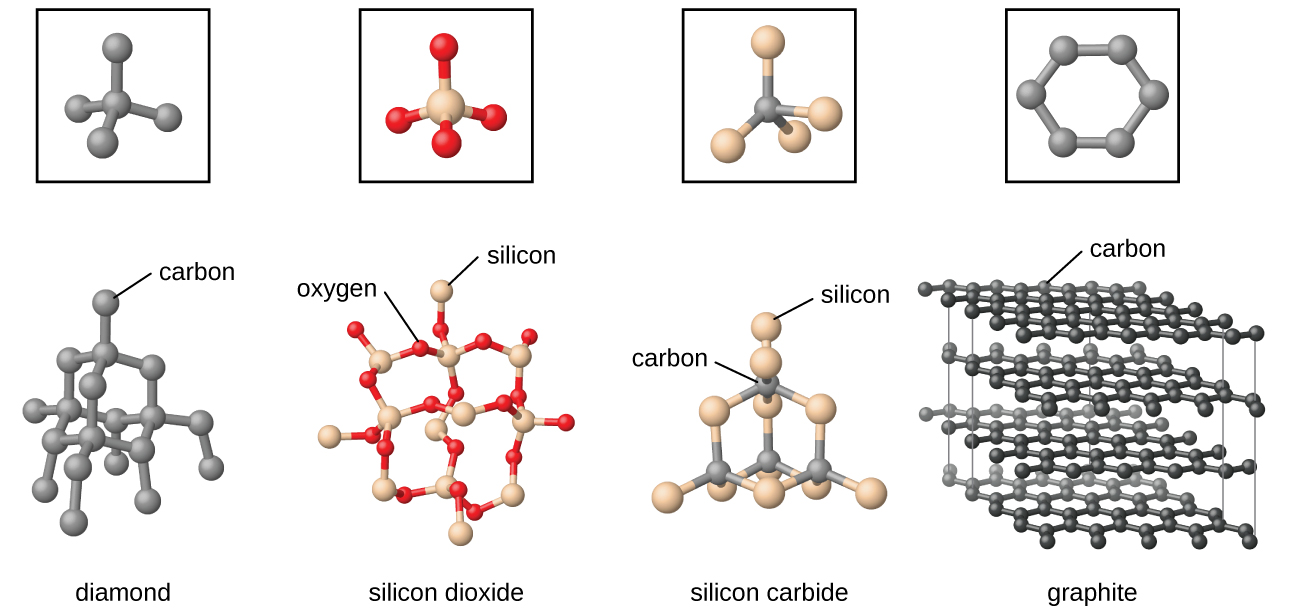
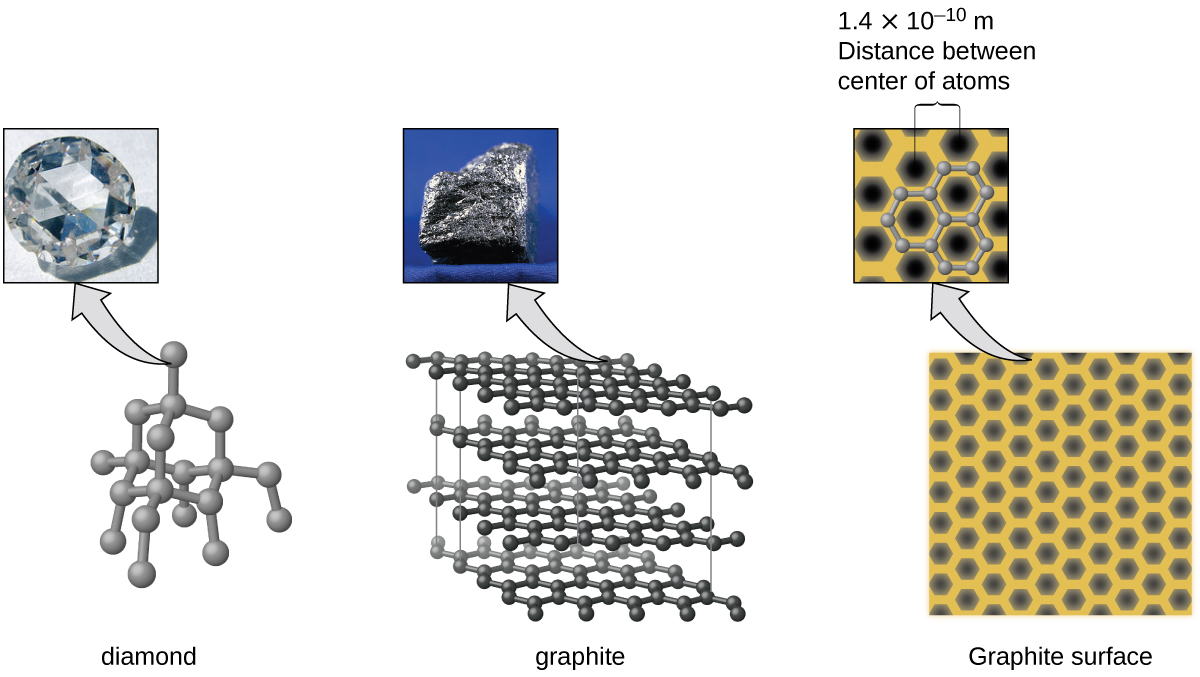
Covalent network solids include crystals of diamond, silicon, some other nonmetals, and some covalent compounds such as silicon dioxide (sand) and silicon carbide (carborundum, the abrasive on sandpaper). Many minerals have networks of covalent bonds. The atoms in these solids are held together by a network of covalent bonds, as shown in Figure 4. To break or to melt a covalent network solid, covalent bonds must be broken. Because covalent bonds are relatively strong, covalent network solids are typically characterized by hardness, strength, and high melting points. For example, diamond is one of the hardest substances known and melts above 3500 °C.
Ionic Solids
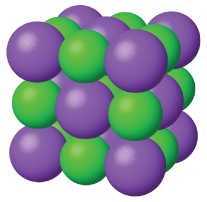
Ionic solids, such as sodium chloride and nickel oxide, are composed of positive and negative ions that are held together by electrostatic attractions, which can be quite strong (Figure 5). Many ionic crystals also have high melting points. This is due to the very strong attractions between the ions—in ionic compounds, the attractions between full charges are (much) larger than those between the partial charges in polar molecular compounds. Recall that you learned about these attractions back in Module 2 when we discussed lattice energy. Although they are hard, they also tend to be brittle, and they shatter rather than bend. Ionic solids do not conduct electricity; however, they do conduct when molten or dissolved because their ions are free to move. Many simple compounds formed by the reaction of a metallic element with a nonmetallic element are ionic.
Molecular Solids
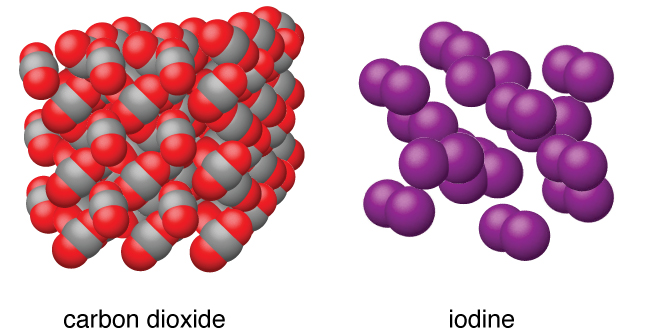
Molecular solids, such as ice, sucrose (table sugar), and iodine, as shown in Figure 6, are composed of neutral molecules. The strengths of the attractive forces between the units present in different crystals vary widely, as indicated by the melting points of the crystals. Small symmetrical molecules (nonpolar molecules), such as H2, N2, O2, and F2, have weak attractive forces and form molecular solids with very low melting points (below −200 °C). Substances consisting of larger, nonpolar molecules have larger attractive forces and melt at higher temperatures. Molecular solids composed of molecules with permanent dipole moments (polar molecules) melt at still higher temperatures. Examples include ice (melting point, 0 °C) and table sugar (melting point, 185 °C).
Properties of Solids
A crystalline solid, like those listed in Table 1, has a precise melting temperature because each atom or molecule of the same type is held in place with the same forces or energy. Thus, the attractions between the units that make up the crystal all have the same strength and all require the same amount of energy to be broken. The gradual softening of an amorphous material differs dramatically from the distinct melting of a crystalline solid. This results from the structural nonequivalence of the molecules in the amorphous solid. Some forces are weaker than others, and when an amorphous material is heated, the weakest intermolecular attractions break first. As the temperature is increased further, the stronger attractions are broken. Thus amorphous materials soften over a range of temperatures.
| Type of Solid | Type of Particles | Type of Attractions | Properties | Examples |
|---|---|---|---|---|
| ionic | ions | ionic bonds | hard, brittle, conducts electricity as a liquid but not as a solid, high to very high melting points | NaCl, Al2O3 |
| metallic | atoms of electropositive elements | metallic bonds | shiny, malleable, ductile, conducts heat and electricity well, variable hardness and melting temperature | Cu, Fe, Ti, Pb, U |
| covalent network | atoms of electronegative elements | covalent bonds | very hard, not conductive, very high melting points | C (diamond), SiO2, SiC |
| molecular | molecules (or atoms) | IMFs | variable hardness, variable brittleness, not conductive, low melting points | H2O, CO2, I2, C12H22O11 |
| Table 1. Types of Crystalline Solids and Their Properties | ||||
Chemistry in Real Life: Graphene—Material of the Future
Carbon is an essential element in our world. The unique properties of carbon atoms allow the existence of carbon-based life forms such as ourselves. Carbon forms a huge variety of substances that we use on a daily basis, including those shown in Figure 7. You may be familiar with diamond and graphite, the two most common allotropes of carbon. (Allotropes are different structural forms of the same element.) Diamond is one of the hardest-known substances, whereas graphite is soft enough to be used as pencil lead. These very different properties stem from the different arrangements of the carbon atoms in the different allotropes.
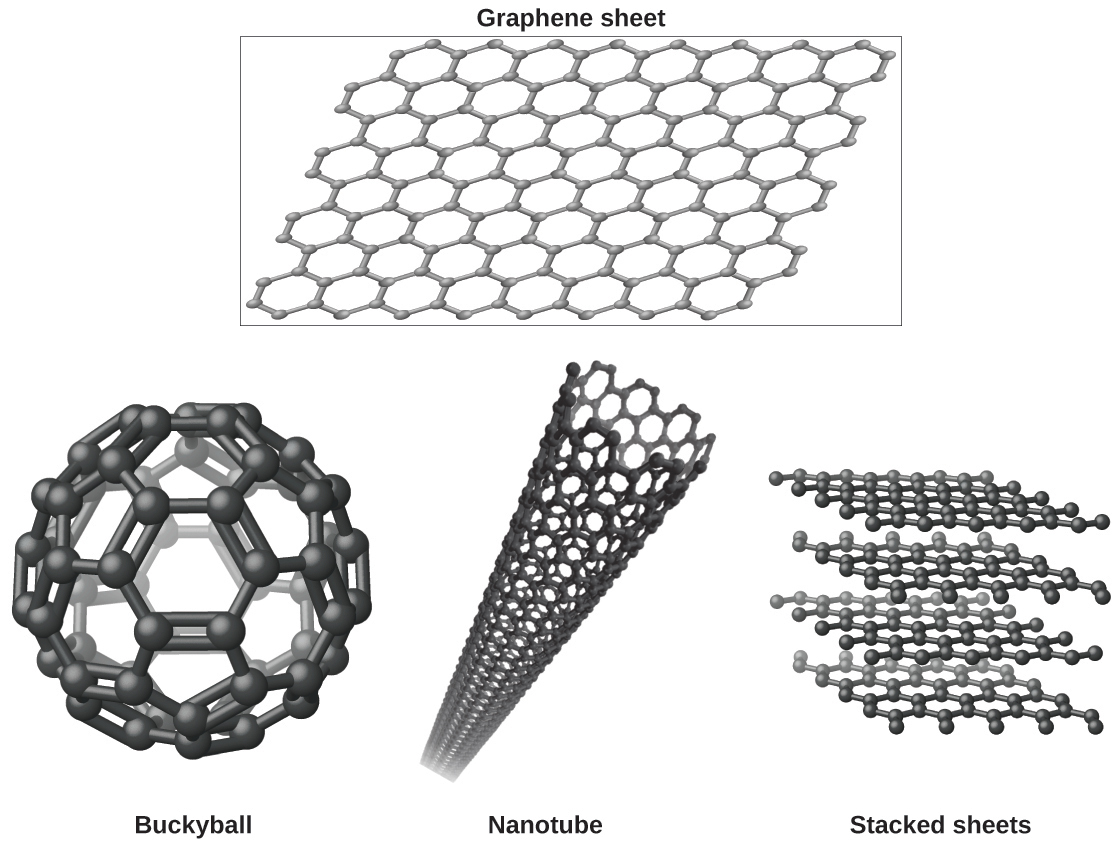
You may be less familiar with a recently discovered form of carbon: graphene. Graphene was first isolated in 2004 by using tape to peel off thinner and thinner layers from graphite. It is essentially a single sheet (one atom thick) of graphite. Graphene, illustrated in Figure 8, is not only strong and lightweight, but it is also an excellent conductor of electricity and heat. These properties may prove very useful in a wide range of applications, such as vastly improved computer chips and circuits, better batteries and solar cells, and stronger and lighter structural materials. The 2010 Nobel Prize in Physics was awarded to Andre Geim and Konstantin Novoselov for their pioneering work with graphene.
Key Concepts and Summary
Some substances form crystalline solids consisting of particles in a very organized structure; others form amorphous (noncrystalline) solids with an internal structure that is not ordered. The main types of crystalline solids are ionic solids, metallic solids, covalent network solids, and molecular solids. The properties of the different kinds of crystalline solids are due to the types of particles of which they consist, the arrangements of the particles, and the strengths of the attractions between them. Because their particles experience identical attractions, crystalline solids have distinct melting temperatures; the particles in amorphous solids experience a range of interactions, so they soften gradually and melt over a range of temperatures.
Glossary
- amorphous solid
- (also, noncrystalline solid) solid in which the particles lack an ordered internal structure
- covalent network solid
- solid whose particles are held together by covalent bonds
- crystalline solid
- solid in which the particles are arranged in a definite repeating pattern
- ionic solid
- solid composed of positive and negative ions held together by strong electrostatic attractions
- metallic solid
- solid composed of metal atoms
- molecular solid
- solid composed of neutral molecules held together by intermolecular forces of attraction
Chemistry End of Section Exercises
- At very low temperatures oxygen, O2, freezes and forms a crystalline solid. Which best describes these crystals?
- ionic
- covalent network
- metallic
- amorphous
- molecular crystals
- Explain why ice, which is a crystalline solid, has a melting temperature of 0 °C, whereas butter, which is an amorphous solid, softens over a range of temperatures.
- Identify the type of crystalline solid (metallic, network covalent, ionic, or molecular) formed by each of the following substances:
- CaCl2
- SiC
- N2
- Fe
- C (graphite)
- CH3CH2CH2CH3
- HCl
- NH4NO3
- K3PO4
- Classify each substance in the table as either a metallic, ionic, molecular, or covalent network solid:
Substance Appearance Melting Point Electrical Conductivity Solubility in Water X lustrous, malleable 1500 °C high insoluble Y soft, yellow 113 °C none insoluble Z hard, white 800 °C only if melted/dissolved soluble Table 2. - Classify each substance in the table as either a metallic, ionic, molecular, or covalent network solid:
Substance Appearance Melting Point Electrical Conductivity Solubility in Water X brittle, white 800 °C only if melted/dissolved soluble Y shiny, malleable 1100 °C high insoluble Z hard, colorless 3550 °C none insoluble Table 3. - Substance A is shiny, conducts electricity well, and melts at 975 °C. Substance A is likely a(n):
- ionic solid
- metallic solid
- molecular solid
- covalent network solid
- Substance B is hard, does not conduct electricity, and melts at 1200 °C. Substance B is likely a(n):
- ionic solid
- metallic solid
- molecular solid
- covalent network solid
Answers to Chemistry End of Section Exercises
- (e)
- Ice has a crystalline structure stabilized by hydrogen bonding. These intermolecular forces are of comparable strength and thus require the same amount of energy to overcome. As a result, ice melts at a single temperature and not over a range of temperatures. The various, very large molecules that compose butter experience varied van der Waals attractions of various strengths that are overcome at various temperatures, and so the melting process occurs over a wide temperature range.
- (a) ionic; (b) covalent network; (c) molecular; (d) metallic; (e) covalent network; (f) molecular; (g) molecular; (h) ionic; (i) ionic
- X = metallic; Y = molecular; Z = ionic
- X = ionic; Y = metallic; Z = covalent network
- (b)
- (d)

