3 Classification of Matter (M1Q3)
Introduction
Learning Objectives for Classification of Matter
- Classify matter as elements, atoms, compounds, and molecules.
| Atoms and Molecules | Classifying Matter | - Distinguish between chemical and physical changes.
| Chemical/Physical Properties & Changes |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Atoms and Molecules
An atom is the smallest particle of an element that has the properties of that element and can enter into a chemical combination. Consider the element gold, for example. Imagine cutting a gold nugget in half, then cutting one of the halves in half, and repeating this process until a piece of gold remained that was so small that it could not be cut in half (regardless of how tiny your knife may be). This minimally sized piece of gold is an atom (from the Greek atomos, meaning “indivisible”) (Figure 1). This atom would no longer be gold if it were divided any further.
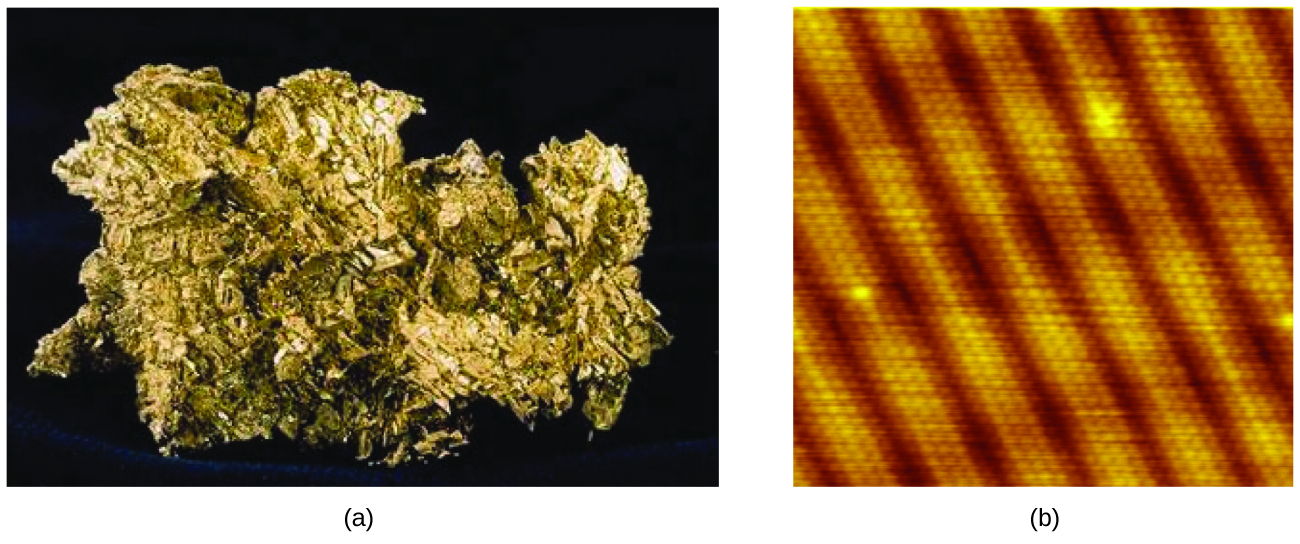
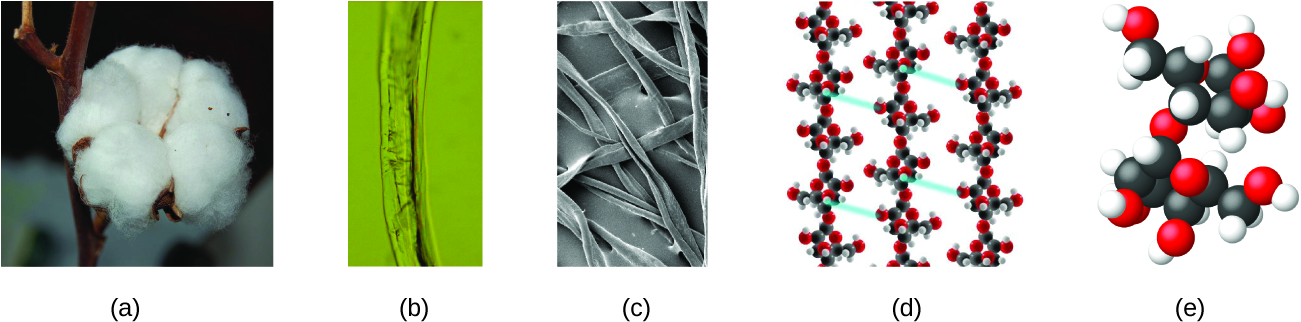
An atom is so small that its size is difficult to imagine. One of the smallest things we can see with our unaided eye is a single thread of a spider web: These strands are about 1/10,000 of a centimeter (0.0001 cm) in diameter. Although the cross-section of one strand is almost impossible to see without a microscope, it is huge on an atomic scale. A single carbon atom in the web has a diameter of about 0.000000015 centimeter, and it would take about 7000 carbon atoms to span the diameter of the strand. To put this in perspective, if a carbon atom were the size of a dime, the cross-section of one strand would be larger than a football field, which would require about 150 million carbon atom “dimes” to cover it. Figure 2 shows increasingly close microscopic and atomic-level views of ordinary cotton.
An atom is so light that its mass is also difficult to imagine. A billion lead atoms (1,000,000,000 atoms) weigh about 3 × 10−13 grams, a mass that is far too light to be weighed on even the world’s most sensitive balances. It would require over 300,000,000,000,000 lead atoms (300 trillion, or 3 × 1014) to be weighed, and they would weigh only 0.0000001 gram.
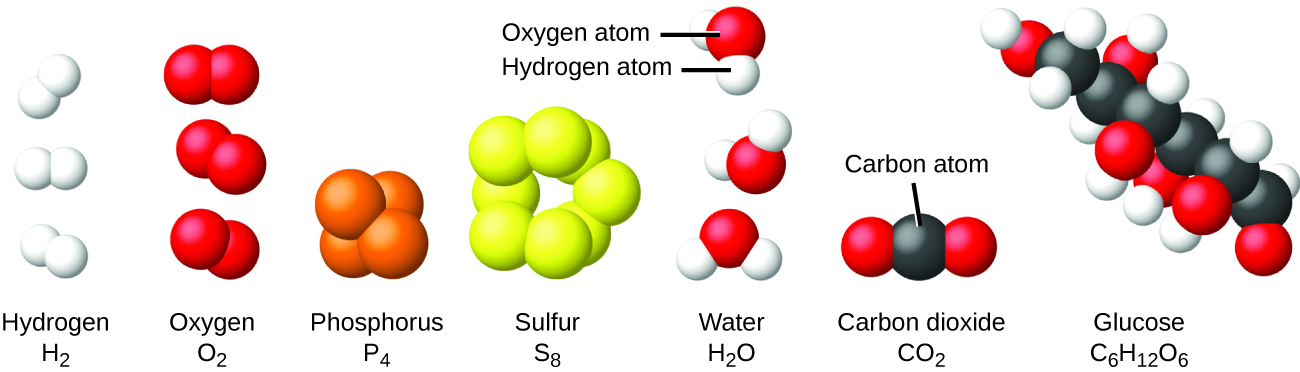
It is rare to find collections of individual atoms. Only a few elements, such as the gases helium, neon, and argon, consist of a collection of individual atoms that move about independently of one another. Other elements, such as the gases hydrogen, nitrogen, oxygen, and chlorine, are composed of units that consist of pairs of atoms (Figure 3). One form of the element phosphorus consists of units composed of four phosphorus atoms. The element sulfur exists in various forms, one of which consists of units composed of eight sulfur atoms. These units are called molecules. A molecule consists of two or more atoms joined by strong forces called chemical bonds. The atoms in a molecule move around as a unit, much like the cans of soda in a six-pack or a bunch of keys joined together on a single key ring. A molecule may consist of two or more identical atoms, as in the molecules found in the elements hydrogen, oxygen, and sulfur, or it may consist of two or more different atoms, as in the molecules found in water. Each water molecule is a unit that contains two hydrogen atoms and one oxygen atom. Each glucose molecule is a unit that contains 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
Like atoms, molecules are incredibly small and light. If an ordinary glass of water were enlarged to the size of the earth, the water molecules inside it would be about the size of golf balls. We represent these molecules using their chemical symbols and subscripts that indicate how many of each atom is present. For example, you have certainly heard water referred to as “H2O”, read as “H two O” (notice how the word “two” refers to the preceding atom). The subscript “2” means that there are 2 hydrogen atoms in a molecule of water. Since oxygen doesn’t have a subscript, it is assumed to be 1. We will return to writing molecule and compound names later, but the examples below provide some practice if you have never seen formulas before (or if it is not fresh in your mind). We assume you are able to understand and complete the examples below as a baseline during these first couple weeks of class. In a later section we will talk in more detail about how to predict formulas, numbers and types of bonds, and chemical properties based on the atoms involved.
Example 1
Writing formulas
Translate the following descriptions into proper chemical formulas. You may wish to consult the periodic table to find the correct symbol for the given elements.
(a) a compound with one potassium and one iodine atom.
(b) a compound someone describes as “C O two”. What are the names of the atoms involved?
(c) a compound someone describes as “L-i three N”. What are the names of the atoms involved?
(d) a compound with 2 atoms of hydrogen and 2 atoms of oxygen
(e) a compound someone describes as “C four H nine O two N S”
Solution
(a) KI
(b) CO2, carbon and oxygen atoms are involved (Astute students may notice that there is also an element on the periodic table called cobalt, with the symbol Co. You will need to be careful about how you communicate whether something is carbon and oxygen (i.e., capital C capital O) or cobalt (i.e., capital C lowercase o). In this example, the space between C and O in the prompt was indicating that these were separate elements, but if you wrote Co2, that would also be fine for this exercise. You will not see questions like this on exams or quizzes. This is just to acclimate you to understanding how people speak in shorthand.)
(c) Li3N (NOT LiN3), lithium and nitrogen atoms are involved (Unlike in (b) above, there is no element with symbol L on the periodic table, so you can deduce that this is the element lithium.)
(d) H2O2
(e) C4H9O2NS, carbon, hydrogen, oxygen, nitrogen and sulfur are involved
Example 2
Writing formulas from ball-and-stick models
The molecules and compounds shown in Figure 3 are space filling models because they do not show the bond between the atoms, and instead only show the space occupied by the atoms. Ball-and-stick models show the atoms as balls and the bonds as sticks. In some structures, you may notice that there are double bonds (i.e., two “sticks”) between atoms. This does not affect how we write the formula, but it does affect the properties of the compound and how it is named. At this point, however, you should just notice that there are double bonds between some atoms and single bonds between others, as we start exploring the structures of compounds.
When displaying molecules, chemists typically use a predictable color scheme. The following color coding system is used in this example:
- white = hydrogen
- black = carbon
- red = oxygen
- green = chlorine
- yellow = sulfur
- dark blue = nitrogen
- light blue = fluorine
For the following ball-and-stick models, determine how many of each type of atom are present, then write a proper chemical formula. Also include how many single and how many double bonds you see in each structure.
(a) 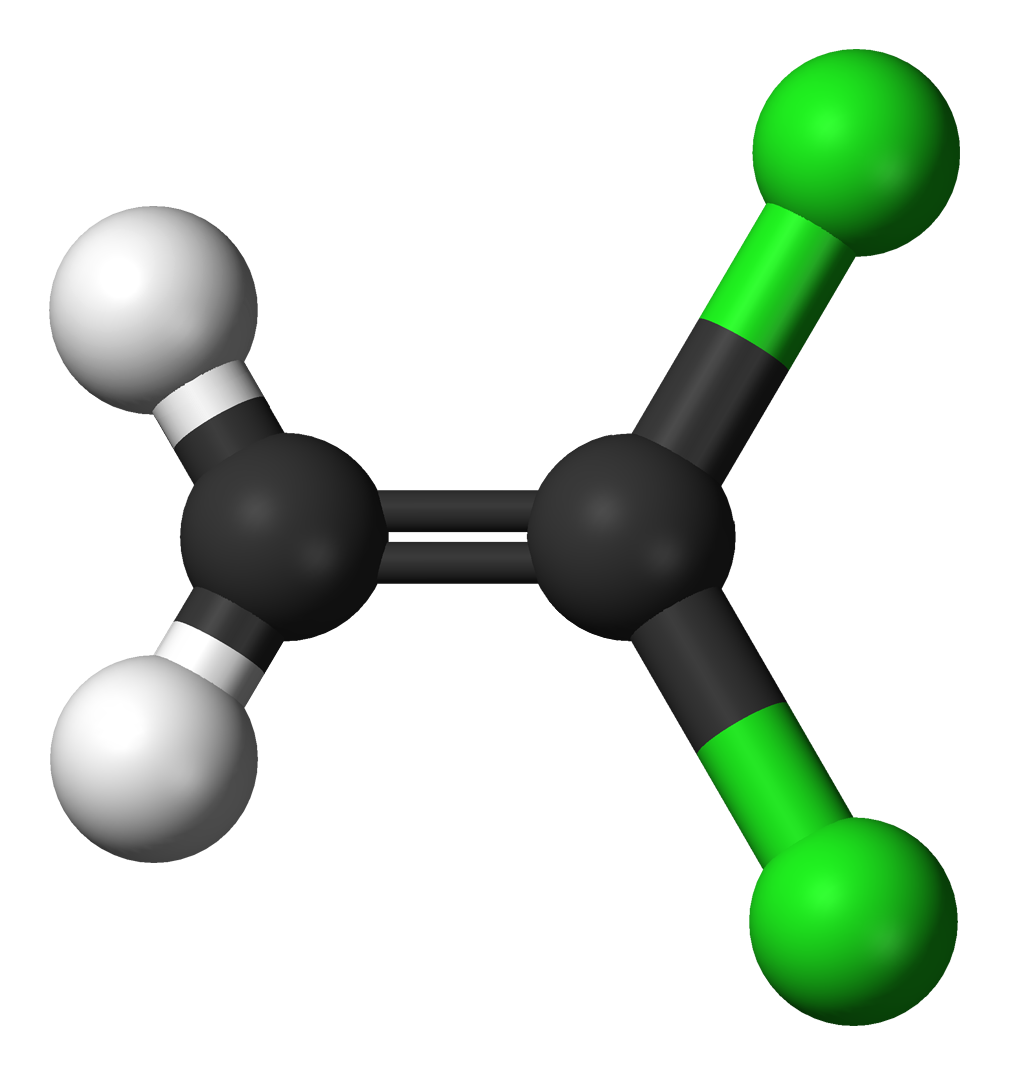
(b) 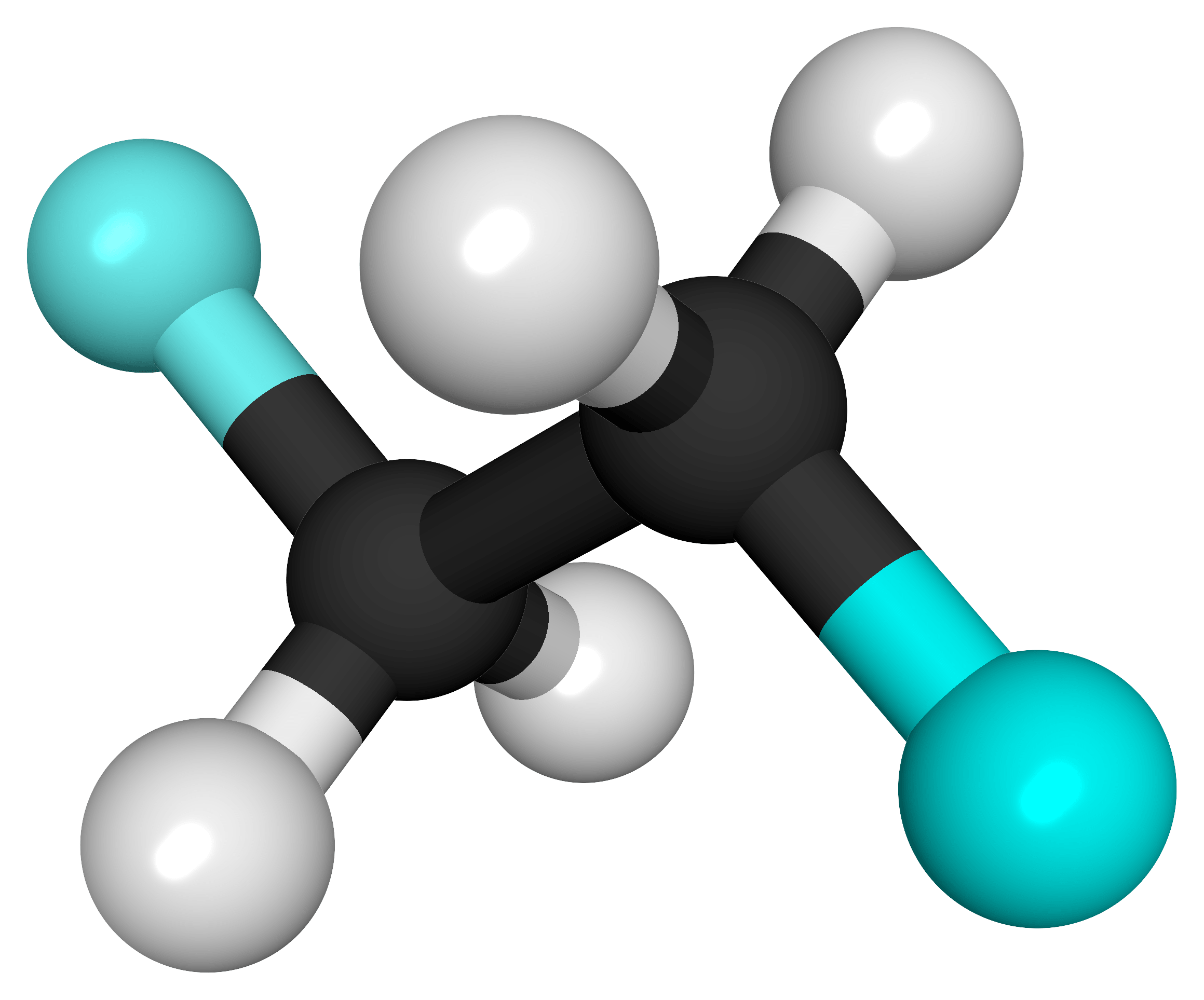
(c) 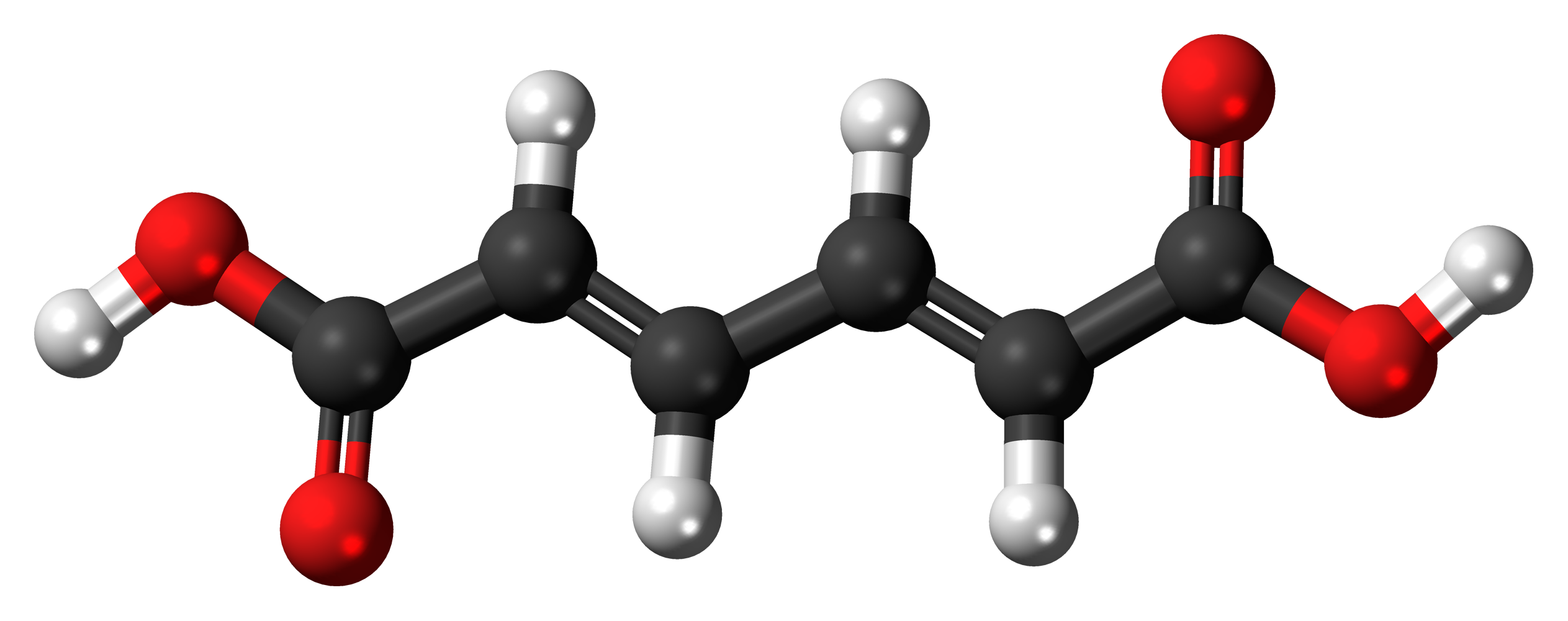
(d) 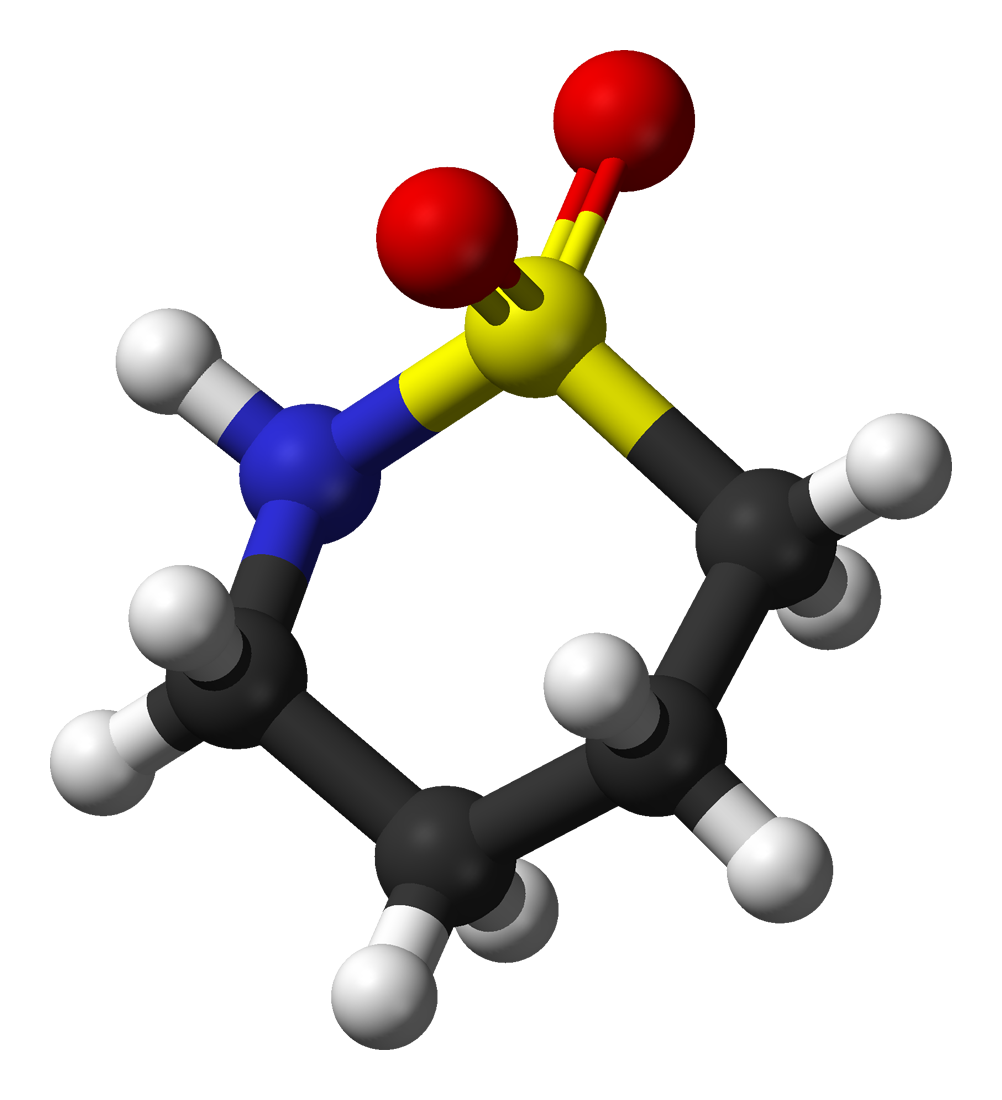
Solution
By convention, the solutions will be written with carbon atoms first, followed by hydrogen atoms.
(a) 2 black spheres = 2 carbon atoms, 2 white spheres = 2 hydrogen atoms, 2 green spheres = 2 chlorine atoms; C2H2Cl2. 1 double bond, 4 single bonds.
(b) 2 black spheres = 2 carbon atoms, 4 white spheres = 4 hydrogen atoms, 2 light blue spheres = 2 fluorine atoms; C2H4F2. 7 single bonds.
(c) 6 black spheres = 6 carbon atoms, 6 white spheres = 6 hydrogen atoms, 4 red spheres = 4 oxygen atoms; C6H6O4. 4 double bonds, 11 single bonds.
(d) 4 black spheres = 4 carbon atoms, 9 white spheres = 9 hydrogen atoms, 1 dark blue sphere = 1 nitrogen atom, 2 red spheres = 2 oxygen atoms, 1 yellow sphere = 1 sulfur atom; C4H9NO2S (notice that we do not need to include a subscript 1 on nitrogen). 2 double bonds, 15 single bonds.
Classifying Matter
We can classify matter into several categories. Two broad categories are mixtures and pure substances. A pure substance has a constant composition. All specimens of a pure substance have exactly the same makeup and properties. Any sample of sucrose (table sugar) consists of 42.1% carbon, 6.5% hydrogen, and 51.4% oxygen by mass. Any sample of sucrose also has the same physical properties, such as melting point, color, and sweetness, regardless of the source from which it is isolated.
We can divide pure substances into two classes: elements and compounds. Pure substances that cannot be broken down into simpler substances by chemical changes are called elements. Iron, silver, gold, aluminum, sulfur, oxygen, and copper are familiar examples of the more than 100 known elements, of which about 90 occur naturally on the earth, and two dozen or so have been created in laboratories.
Eleven elements make up about 99% of the earth’s crust and atmosphere (Table 1). Oxygen constitutes nearly one-half and silicon about one-quarter of the total quantity of these elements. A majority of elements on earth are found in chemical combinations with other elements; about one-quarter of the elements are also found in the free state.
| Element | Symbol | Percent Mass | Element | Symbol | Percent Mass | |
|---|---|---|---|---|---|---|
| oxygen | O | 49.20 | chlorine | Cl | 0.19 | |
| silicon | Si | 25.67 | phosphorus | P | 0.11 | |
| aluminum | Al | 7.50 | manganese | Mn | 0.09 | |
| iron | Fe | 4.71 | carbon | C | 0.08 | |
| calcium | Ca | 3.39 | sulfur | S | 0.06 | |
| sodium | Na | 2.63 | barium | Ba | 0.04 | |
| potassium | K | 2.40 | nitrogen | N | 0.03 | |
| magnesium | Mg | 1.93 | fluorine | F | 0.03 | |
| hydrogen | H | 0.87 | strontium | Sr | 0.02 | |
| titanium | Ti | 0.58 | all others | – | 0.47 | |
| Table 1. Elemental Composition of Earth | ||||||
Pure substances that can be broken down by chemical changes are called compounds. This breakdown may produce either elements or other compounds, or both. Mercury(II) oxide, an orange, crystalline solid, can be broken down by heat into the elements mercury and oxygen (Figure 4). When heated in the absence of air, the compound sucrose is broken down into the element carbon and the compound water. (The initial stage of this process, when the sugar is turning brown, is known as caramelization—this is what imparts the characteristic sweet and nutty flavor to caramel apples, caramelized onions, and caramel). Silver(I) chloride is a white solid that can be broken down into its elements, silver and chlorine, by absorption of light. This property is the basis for the use of this compound in photographic films and photochromic eyeglasses (those with lenses that darken when exposed to light).
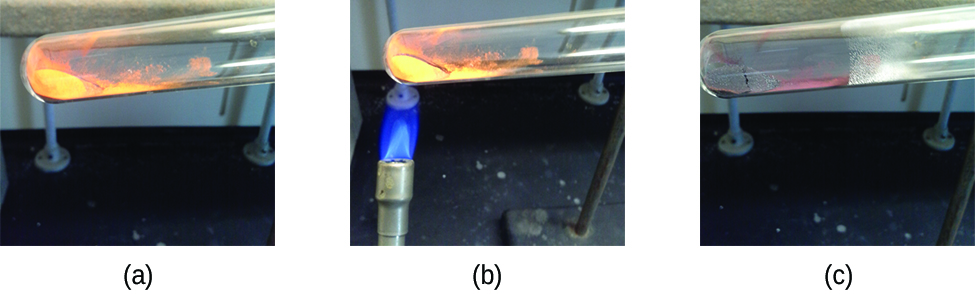

Many compounds break down when heated. This site shows the breakdown of mercury oxide, HgO. You can also view an example of the photochemical decomposition of silver chloride (AgCl), the basis of early photography.
The properties of combined elements are different from those in the free, or uncombined, state. For example, white crystalline sugar (sucrose) is a compound resulting from the chemical combination of the element carbon, which is a black solid in one of its uncombined forms, and the two elements hydrogen and oxygen, which are colorless gases when uncombined. Free sodium, an element that is a soft, shiny, metallic solid, and free chlorine, an element that is a yellow-green gas, combine to form sodium chloride (table salt), a compound that is a white, crystalline solid.
A mixture is composed of two or more types of matter that can be present in varying amounts and can be separated by physical changes, such as evaporation (you will learn more about this later). A mixture with a composition that varies from point to point is called a heterogeneous mixture. Italian dressing is an example of a heterogeneous mixture (Figure 5). Its composition can vary because we can make it from varying amounts of oil, vinegar, and herbs. It is not the same from point to point throughout the mixture—one drop may be mostly vinegar, whereas a different drop may be mostly oil or herbs because the oil and vinegar separate and the herbs settle. Other examples of heterogeneous mixtures are chocolate chip cookies (we can see the separate bits of chocolate, nuts, and cookie dough) and granite (we can see the quartz, mica, feldspar, and more).
A homogeneous mixture, also called a solution, exhibits a uniform composition and appears visually the same throughout. An example of a solution is a sports drink, consisting of water, sugar, coloring, flavoring, and electrolytes mixed together uniformly (Figure 5). Each drop of a sports drink tastes the same because each drop contains the same amounts of water, sugar, and other components. Note that the composition of a sports drink can vary—it could be made with somewhat more or less sugar, flavoring, or other components, and still be a sports drink. Other examples of homogeneous mixtures include air, maple syrup, gasoline, and a solution of salt in water.

Decomposition of Water / Production of Hydrogen
Water consists of the elements hydrogen and oxygen combined in a 2 to 1 ratio. Water can be broken down into hydrogen and oxygen gases by the addition of energy. One way to do this is with a battery or power supply, as shown in Figure 6.
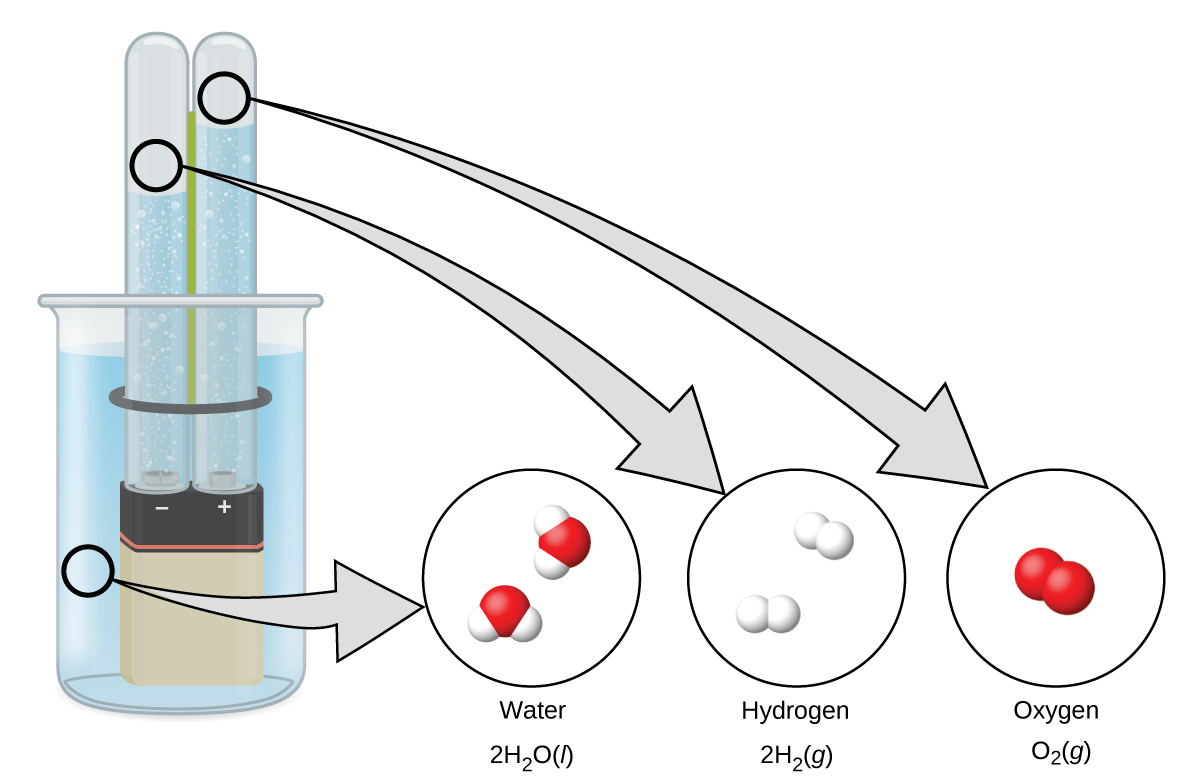
The breakdown of water involves a rearrangement of the atoms in water molecules into different molecules, each composed of two hydrogen atoms and two oxygen atoms, respectively. Two water molecules form one oxygen molecule and two hydrogen molecules. The representation for what occurs, 2 H2O(l) → 2 H2(g) + O2(g), will be explored in more depth in later sections.
The two gases produced have distinctly different properties. Oxygen is not flammable but is required for combustion of a fuel, and hydrogen is highly flammable and a potent energy source.
Chemistry in Real Life: Chemistry of Cell Phones
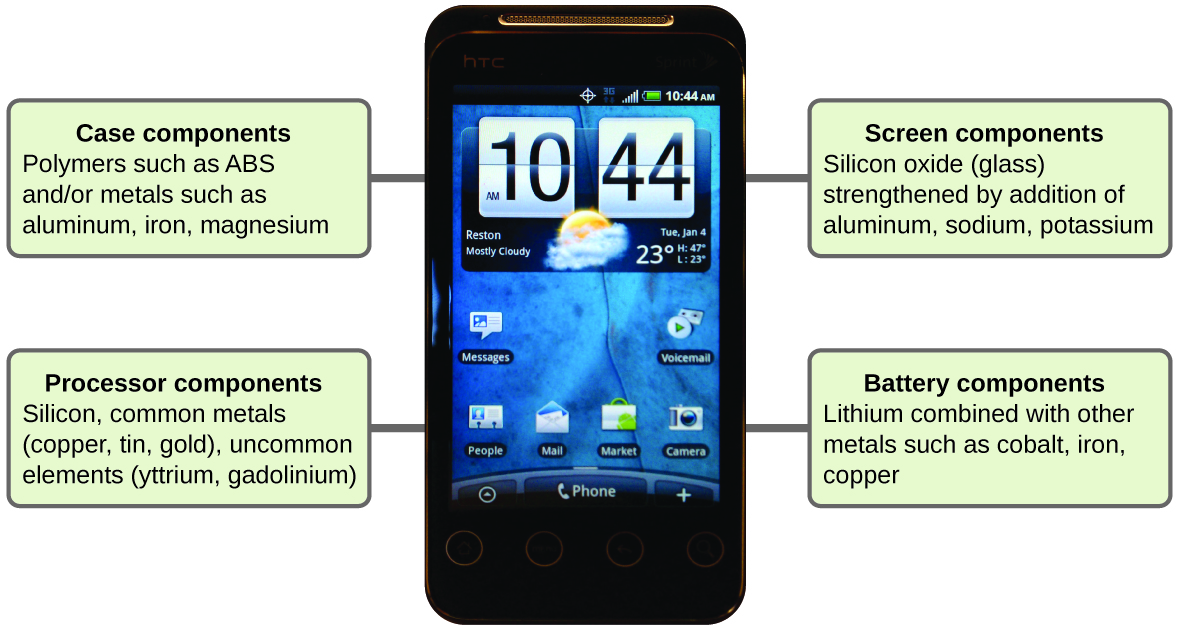
Imagine how different your life would be without cell phones (Figure 7) and other smart devices. Cell phones are made from numerous chemical substances, which are extracted, refined, purified, and assembled using an extensive and in-depth understanding of chemical principles. About 30% of the elements that are found in nature are found within a typical smart phone. The case/body/frame consists of a combination of sturdy, durable polymers comprised primarily of carbon, hydrogen, oxygen, and nitrogen [acrylonitrile butadiene styrene (ABS) and polycarbonate thermoplastics], and light, strong, structural metals, such as aluminum, magnesium, and iron. The display screen is made from a specially toughened glass (silica glass strengthened by the addition of aluminum, sodium, and potassium) and coated with a material to make it conductive (such as indium tin oxide). The circuit board uses a semiconductor material (usually silicon); commonly used metals like copper, tin, silver, and gold; and more unfamiliar elements such as yttrium, praseodymium, and gadolinium. The battery relies upon lithium ions and a variety of other materials, including iron, cobalt, copper, polyethylene oxide, and polyacrylonitrile.
Chemical/Physical Properties & Changes
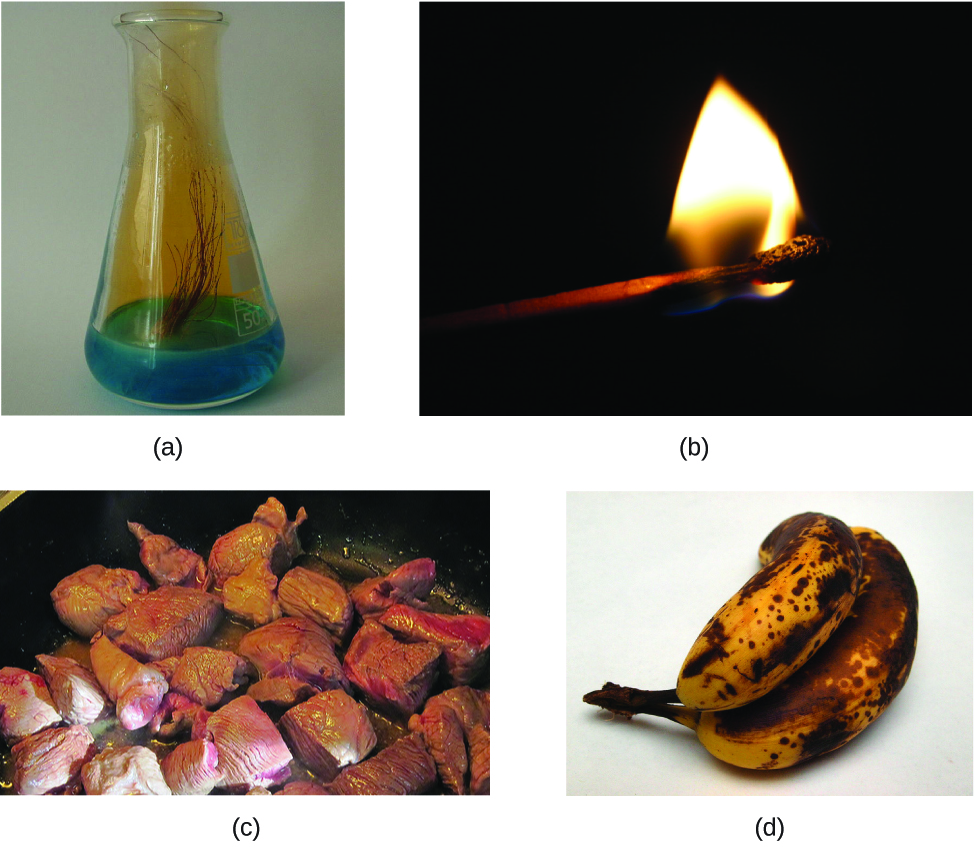
The characteristics that enable us to distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. We can observe some physical properties, such as density and color, without changing the physical state of the matter observed. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, can only be observed as matter undergoes a physical change. A physical change is a change in the state or properties of matter without any accompanying change in its chemical composition (the identities of the substances contained in the matter). We observe a physical change when wax melts, when sugar dissolves in coffee, and when steam condenses into liquid water (Figure 8). Other examples of physical changes include magnetizing and demagnetizing metals (as is done with common anti-theft security tags) and grinding solids into powders (which can sometimes yield noticeable changes in color). In each of these examples, there is a change in the physical state, form, or properties of the substance, but no change in its chemical composition.

The change of one type of matter into another type (or the inability to change) is a chemical property. Examples of chemical properties include flammability, toxicity, acidity, reactivity (many types), and heat of combustion. Iron, for example, combines with oxygen in the presence of water to form rust; chromium does not oxidize (Figure 9). Nitroglycerin is very dangerous because it explodes easily; neon poses almost no hazard because it has little to no reactivity.

Demonstrations: Flammability of gases
Set up. The following video shows balloons filled with various gases. The green balloon contains helium, the yellow balloon contains hydrogen gas (H2) and the red balloon contains a mixture of hydrogen gas (H2) and oxygen gas (O2). A torch is used to ignite the balloons and the flammability of the different gases will be assessed by the amount of sound produced in each experiment.
Prediction. Which balloon do you predict will be the most flammable?
Explanation. The sound produced from the explosions provides a measure of how flammable the gas was. The sound produced is a form of energy released by the reaction. If the gas was extremely flammable, a louder noise was generated. In this video, the red balloon (which contained a mix of hydrogen and oxygen gas) produced the loudest noise and therefore the mixture of these gases was the most flammable. The helium balloon produced the smallest reaction, indicating that it is the least flammable.
To identify a chemical property, we look for a chemical change. A chemical change always produces one or more types of matter that differ from the matter present before the change. The formation of rust is a chemical change because rust is a different kind of matter than the iron, oxygen, and water present before the rust formed. The explosion of nitroglycerin is a chemical change because the gases produced are very different kinds of matter from the original substance. Other examples of chemical changes include reactions that are performed in a lab (such as copper reacting with nitric acid), all forms of combustion (burning), and food being cooked, digested, or rotting (Figure 10).
Hazard Diamond
You may have seen the symbol shown in Figure 11 on containers of chemicals in a laboratory or workplace. Sometimes called a “fire diamond” or “hazard diamond,” this chemical hazard diamond provides valuable information that briefly summarizes the various dangers of which to be aware when working with a particular substance.
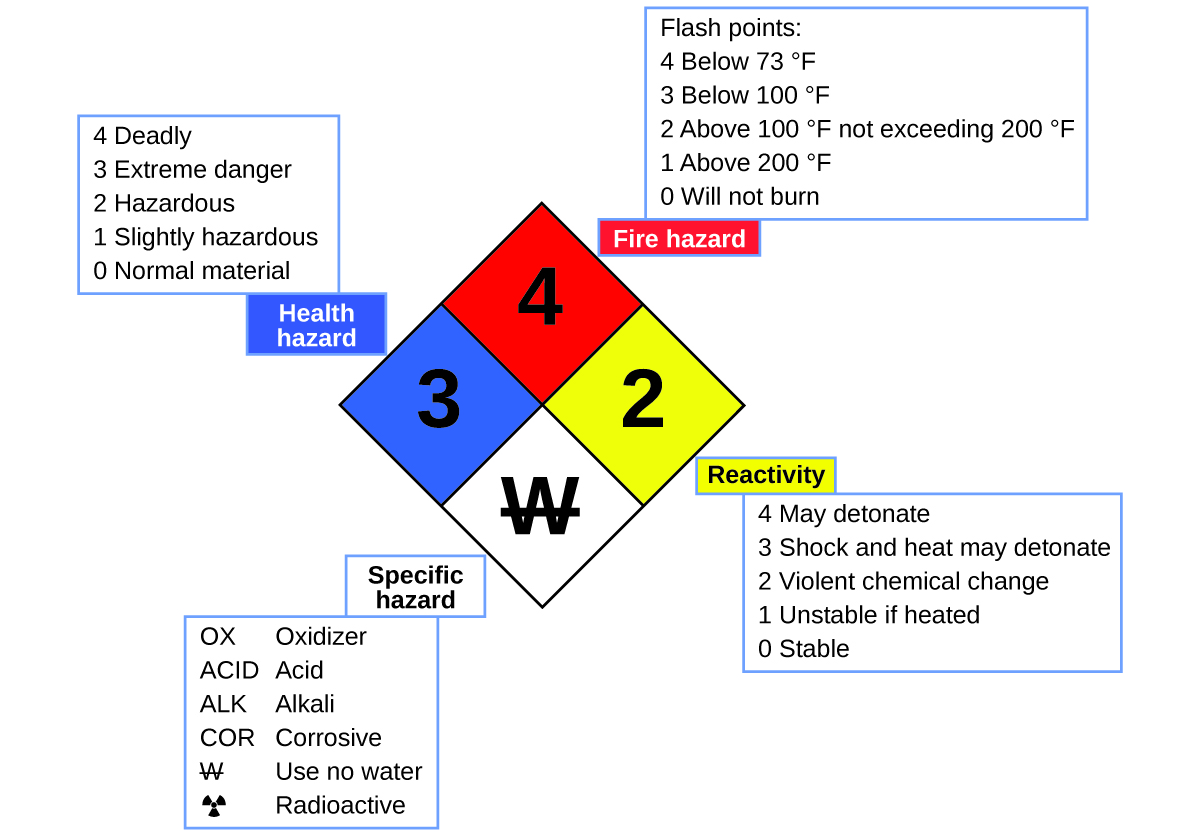
The National Fire Protection Agency (NFPA) 704 Hazard Identification System was developed by NFPA to provide safety information about certain substances. The system details flammability, reactivity, health, and other hazards. Within the overall diamond symbol, the top (red) diamond specifies the level of fire hazard (temperature range for flash point). The blue (left) diamond indicates the level of health hazard. The yellow (right) diamond describes reactivity hazards, such as how readily the substance will undergo detonation or a violent chemical change. The white (bottom) diamond points out special hazards, such as if it is an oxidizer (which allows the substance to burn in the absence of air/oxygen), undergoes an unusual or dangerous reaction with water, is corrosive, acidic, alkaline, a biological hazard, radioactive, and so on. Each hazard is rated on a scale from 0 to 4, with 0 being no hazard and 4 being extremely hazardous.
While many elements differ dramatically in their chemical and physical properties, some elements have similar properties. We can identify sets of elements that exhibit common behaviors. For example, many elements conduct heat and electricity well, whereas others are poor conductors. These properties can be used to sort the elements into three classes: metals (elements that conduct well), nonmetals (elements that conduct poorly), and metalloids (elements that have properties of both metals and nonmetals).
The periodic table is a table of elements that places elements with similar properties close together (Figure 12). You will learn more about the periodic table as you continue your study of chemistry.
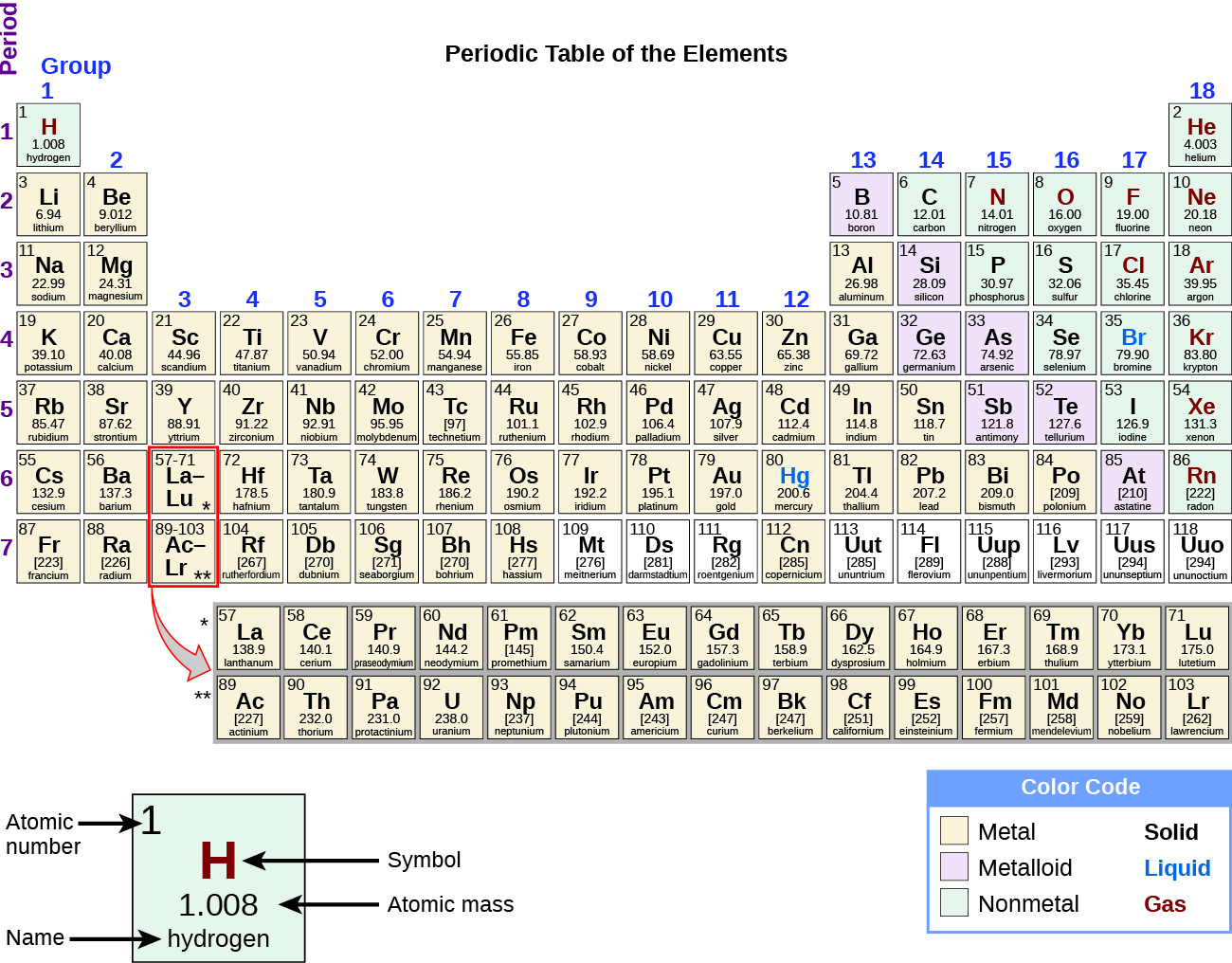
Demonstration: Periodic trend of alkali metal reactivity with water
In this demonstration, we explore the reactivity of the alkali metals (those in the first column of the periodic table above) with water. A small amount of phenolphthalein indicator is added to the water. This indicator will turn from colorless to pink when the water becomes basic (contains more hydroxide ions). Then a small amount of lithium, sodium, or potassium is added to assess their reactivity.
This video shows that all three alkali metals react strongly with water. These alkali metals are arranged in column 1 of the periodic table. We saw that as we go down the column (as we go from Li to Na to K), the reaction was much more vigorous. While Li reacted with water and produced bubbles, when K was added to water, a fire started because it was so reactive! In all three reactions, you also saw that the solution turned from colorless to pink, which indicated that as the reaction occurred, the solution was becoming more basic. In fact, as the reaction occurred, LiOH, NaOH, or KOH was formed, resulting in a more basic solution.
Key Concepts and Summary
Atoms are the smallest particle of an element that still have the properties of an element. A molecule is a combination of atoms. Matter can be classified as either pure substances (elements and compounds) or mixtures (homogeneous and heterogeneous mixtures), depending on their characteristics.
All substances have distinct physical and chemical properties, and may undergo physical or chemical changes. Physical properties, such as hardness and boiling point, and physical changes, such as melting or freezing, do not involve a change in the composition of matter. Chemical properties, such flammability and acidity, and chemical changes, such as rusting, involve production of matter that differs from that present beforehand.
Glossary
- atom
- smallest particle of an element that can enter into a chemical combination
- chemical change
- change producing a different kind of matter from the original kind of matter
- chemical property
- behavior that is related to the change of one kind of matter into another kind of matter
- compound
- pure substance that can be decomposed into two or more elements
- element
- substance that is composed of a single type of atom; a substance that cannot be decomposed by a chemical change
- molecule
- bonded collection of two or more atoms of the same or different elements
- physical change
- change in the state or properties of matter that does not involve a change in its chemical composition
- physical property
- characteristic of matter that is not associated with any change in its chemical composition
- pure substance
- homogeneous substance that has a constant composition
Chemistry End of Section Exercises
Classifying Matter
- How does an element differ from a compound? How are they similar?
- How do molecules of elements and molecules of compounds differ? In what ways are they similar?
- Classify each of the following as an element or a compound:
- copper
- water
- nitrogen
- sulfur
- sucrose
- a substance composed of molecules each of which contains two iodine atoms
- A sulfur atom and a sulfur molecule are not identical. What is the difference?
- How are the molecules in oxygen gas, the molecules in hydrogen gas, and water molecules similar? How do they differ?
- Matter is everywhere around us. Make a list by name of fifteen different kinds of matter that you encounter every day. Your list should include (and label at least one example of each) the following: a solid, a liquid, a gas, an element, a compound, and a pure substance.
Chemical/Physical Properties & Changes
- Classify the six underlined properties in the following paragraph as chemical or physical:
Fluorine is a pale yellow gas that reacts with most substances. The free element melts at −220 °C and boils at −188 °C. Finely divided metals burn in fluorine with a bright flame. Nineteen grams of fluorine will react with 1.0 gram of hydrogen.
- Classify each of the following changes as physical or chemical:
- condensation of steam
- burning of gasoline
- souring of milk
- dissolving of sugar in water
- melting of gold
- The volume of a sample of oxygen gas changed from 10 mL to 11 mL as the temperature changed. Is this a chemical or physical change?
- A 2.0-liter volume of hydrogen gas combined with 1.0 liter of oxygen gas to produce 2.0 liters of water vapor. Does oxygen undergo a chemical or physical change?
Answers to Chemistry End of Section Exercises
- Differences: an element cannot be divided further, but a compound can since it contains more than two elements. Similarities: Both can be very abundant naturally, both can be classified as pure substances.
- Molecules of elements contain only one type of atom; molecules of compounds contain two or more types of atoms. They are similar in that both are comprised of two or more atoms chemically bonded together.
- (a) element; (b) compound; (c) element; (d) element; (e) compound; (f) element
- A sulfur molecule is multiple atoms of sulfur.
- In each case, a molecule consists of two or more combined atoms. They differ in that the types of atoms change from one substance to the next.
- Some examples: Solid silver, Water, Oxygen Gas, Nitrogen Gas, Water Vapor, Iron, Table Salt/NaCl, Tums/CaCl, Titanium (jewelry), Aluminum, Plastic (compound), Brass (compound), glass, gas for your car (compound), ice.
- physical, chemical, physical, physical, chemical, chemical
- (a) physical; (b) chemical; (c) chemical; (d) physical; (e) physical
- physical change
- chemical change

