51 Valence Bond Theory and Resonance (M9Q4)
Introduction
Learning Objectives for Valence Bond Theory and Resonance
- Apply valence bond theory to describe and illustrate sigma and pi bonds in molecules, and to rationalize cis/trans isomerization and/or resonance.
| Sigma and Pi Bonds | Unhybridized p Orbitals and Resonance |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Sigma (σ) and Pi (π) Bonds
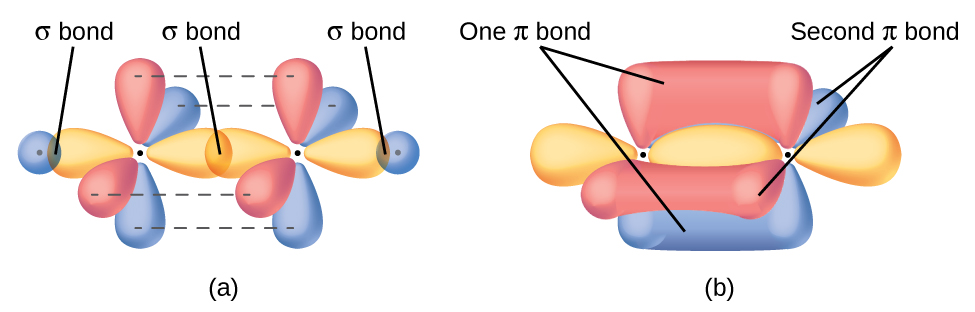
The overlap of two s orbitals (as in H2), the overlap of an s orbital and a p orbital (as in HCl), and the end-to-end overlap of two p orbitals (as in Cl2) all produce sigma bonds (σ bonds), as illustrated in Figure 1. A σ bond is a covalent bond in which the electron density is concentrated in the region along the internuclear axis; that is, a line between the nuclei would pass through the center of the overlap region. Single bonds in Lewis structures are described as σ bonds in valence bond theory.
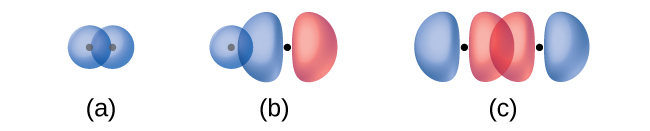
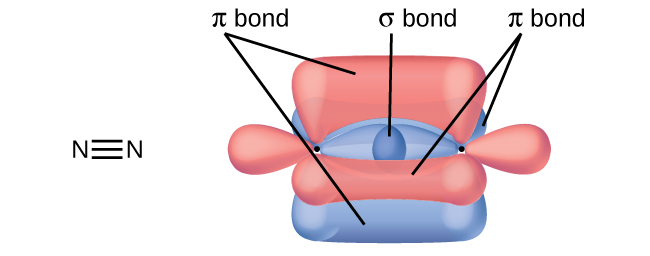
A pi bond (π bond) is a type of covalent bond that results from the side-by-side overlap of two p orbitals, as illustrated in Figure 2. In a π bond, the regions of orbital overlap lie on opposite sides of the internuclear axis. Along the axis itself, there is a node, that is, a plane with no probability of finding an electron.
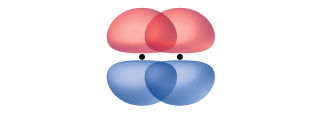
While all single bonds are σ bonds, multiple bonds consist of both σ and π bonds. As the Lewis structures suggest, O2 contains a double bond, and N2 contains a triple bond. The double bond consists of one σ bond and one π bond, and the triple bond consists of one σ bond and two π bonds. Between any two atoms, the first bond formed will always be a σ bond, but there can only be one σ bond in any one location. In any multiple bond, there will be one σ bond, and the remaining one or two bonds will be π bonds. These bonds are described in more detail later in this section.
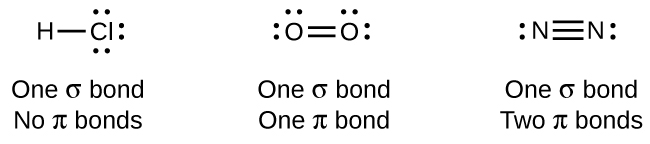
An average carbon-carbon single bond is 356 kJ/mol, while in a carbon-carbon double bond, the π bond increases the bond strength by 242 kJ/mol. Adding an additional π bond causes a further increase of 215 kJ/mol. We can see a similar pattern when we compare other σ and π bonds. Thus, each individual π bond is generally weaker than a corresponding σ bond between the same two atoms. In a σ bond, there is a greater degree of orbital overlap than in a π bond.
Example 1
Counting σ and π Bonds
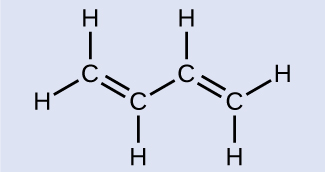
Butadiene, C6H6, is used to make synthetic rubber. Identify the number of σ and π bonds depicted by the most important resonance structure of this molecule, shown above.
Solution
There are six σ C–H bonds and one σ C–C bond, for a total of seven from the single bonds. There are two double bonds that each have a π bond in addition to the σ bond. This gives a total nine σ and two π bonds overall.
Check Your Learning
Identify each illustration as depicting a σ or π bond:
(a) side-by-side overlap of a 4p and a 2p orbital
(b) end-to-end overlap of a 4p and 4p orbital
(c) end-to-end overlap of a 4p and a 2p orbital
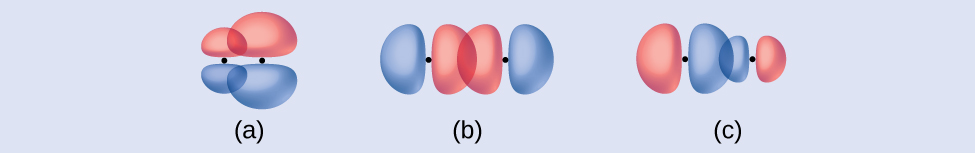
Answer:
(a) is a π bond with a node along the axis connecting the nuclei while (b) and (c) are σ bonds that overlap along the internuclear axis.
Multiple Bonds, Unhybridized p Orbitals and Resonance Structures
The hybrid orbital model appears to account well for the geometry of molecules involving single covalent bonds. Is it also capable of describing molecules containing double and triple bonds? We have already discussed that multiple bonds consist of σ and π bonds. Next we can consider how we visualize these components and how they relate to hybrid orbitals. The Lewis structure of ethene, C2H4, shows us that each carbon atom is surrounded by one other carbon atom and two hydrogen atoms.
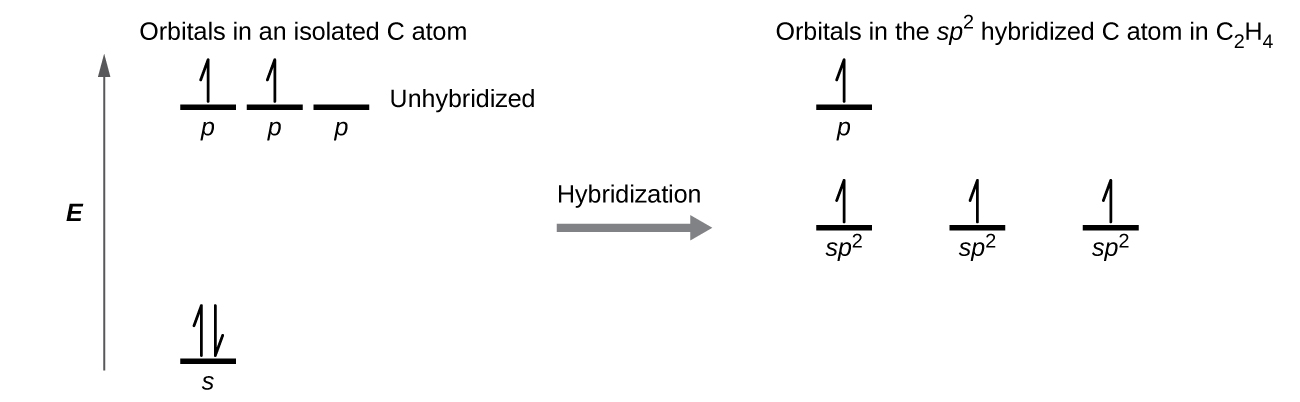
The three bonding regions form a trigonal planar electron-pair geometry. Thus we expect the σ bonds from each carbon atom are formed using a set of sp2 hybrid orbitals that result from hybridization of two 2p orbitals and the 2s orbital (Figure 3). These orbitals form the C–H single bonds and the σ bond in the C=C double bond (Figure 4). The π bond in the C=C double bond results from the overlap of the third (remaining) 2p orbital on each carbon atom that is not involved in hybridization. This unhybridized p orbital (lobes shown in red and blue in Figure 4) is perpendicular to the plane of the sp2 hybrid orbitals. Thus the unhybridized 2p orbitals overlap in a side-by-side fashion, above and below the internuclear axis (Figure 4) and form a π bond.
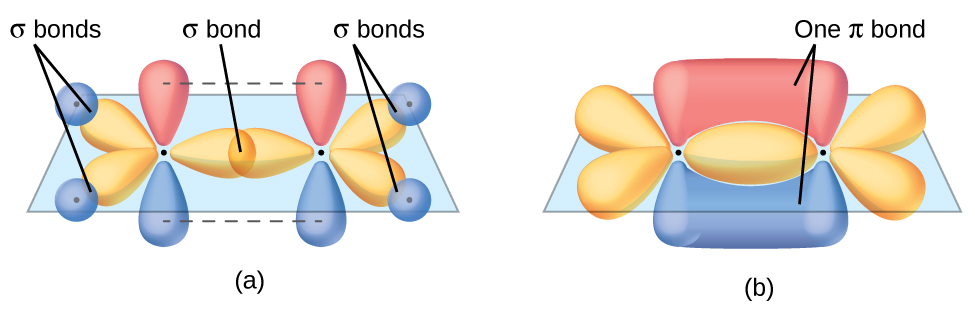
In an ethene molecule, the four hydrogen atoms and the two carbon atoms are all in the same plane. If the two planes of sp2 hybrid orbitals tilted relative to each other, the p orbitals would not be oriented to overlap efficiently to create the π bond. The planar configuration for the ethene molecule occurs because it is the most stable bonding arrangement. This is a significant difference between σ and π bonds; rotation around single (σ) bonds occurs easily because the end-to-end orbital overlap does not depend on the relative orientation of the orbitals on each atom in the bond. In other words, rotation around the internuclear axis does not change the extent to which the σ bonding orbitals overlap because the bonding electron density is symmetric about the axis. Rotation about the internuclear axis is much more difficult for multiple bonds; however, this would drastically alter the off-axis overlap of the π bonding orbitals, essentially breaking the π bond.
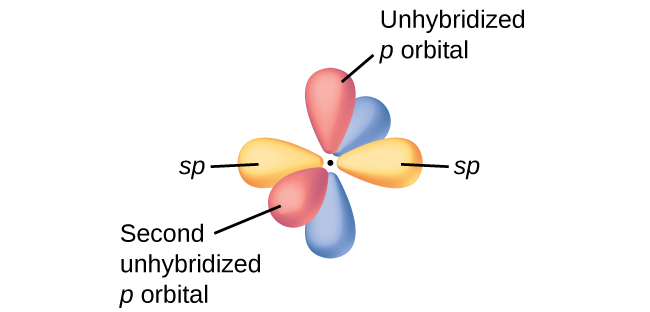
In molecules with sp hybrid orbitals, two unhybridized p orbitals remain on the atom (Figure 5). We find this situation in acetylene, H−C≡C−H, which is a linear molecule. The sp hybrid orbitals of the two carbon atoms overlap end to end to form a σ bond between the carbon atoms (Figure 6). The remaining sp orbitals form σ bonds with hydrogen atoms. The two unhybridized p orbitals per carbon are positioned such that they overlap side by side and, hence, form two π bonds. The two carbon atoms of acetylene are thus bound together by one σ bond and two π bonds, giving a triple bond.
Hybridization involves only σ bonds, lone pairs of electrons, and single unpaired electrons (radicals). Structures that account for these features describe the correct hybridization of the atoms. However, many structures also include resonance forms. Remember that different resonance forms are required to represent the molecule when various arrangements of π bonds are possible.
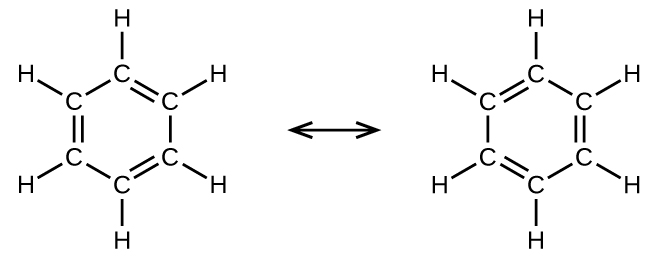
For example, the molecule benzene has two resonance forms (Figure 7). We can use either of these forms to determine that each of the carbon atoms is bonded to three other atoms with no lone pairs, so the correct hybridization is sp2. The electrons in the unhybridized p orbitals form π bonds. Neither resonance structure completely describes the electrons in the π bonds. They are not located in one position or the other, but in reality are delocalized throughout the ring. A simple application of Valence Bond theory does not easily address delocalization. Bonding in molecules with resonance forms is better described by molecular orbital theory (see the next sections) or by invoking hyperconjugation (to learn more, enroll in CHEM 343).
Example 2
Assignment of Hybridization Involving Resonance
Some acid rain results from the reaction of sulfur dioxide with atmospheric water vapor, followed by the formation of sulfuric acid. Sulfur dioxide, SO2, is a major component of volcanic gases as well as a product of the combustion of sulfur-containing coal. What is the hybridization of the S atom in SO2?
Solution
The resonance structures of SO2 are
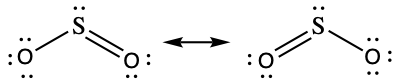
The sulfur atom is surrounded by two bonding regions and one lone pair of electrons in either resonance structure. Therefore, the electron-pair geometry is trigonal planar, and the hybridization of the sulfur atom is sp2.
Check Your Learning
Another acid in acid rain is nitric acid, HNO3, which is produced by the reaction of nitrogen dioxide, NO2, with atmospheric water vapor. What is the hybridization of the nitrogen atom in NO2? (Note: the lone electron on nitrogen occupies a hybridized orbital just as a lone pair would.)
Answer:
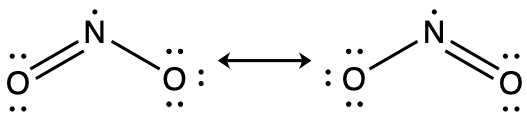
sp2
Key Concepts and Summary
Valence bond theory describes bonding as a consequence of the overlap of two separate atomic orbitals on different atoms that creates a region with one pair of electrons shared between the two atoms. When the orbitals overlap along an axis containing the nuclei, they form a σ bond. When they overlap in a fashion that creates a node along this axis, they form a π bond. Multiple bonds consist of a σ bond located along the axis between two atoms and one or two π bonds. The σ bonds are usually formed by the overlap of hybridized atomic orbitals, while the π bonds are formed by the side-by-side overlap of unhybridized p orbitals. Resonance structures are required when multiple representations of the molecule are required to depict the delocalization of electrons across multiple atoms. This often occurs when there are unhybridized p orbitals on atoms adjacent to atoms involved in π bonds.
Glossary
- node
- plane separating different lobes of orbitals, where the probability of finding an electron is zero
- pi bond (π bond)
- covalent bond formed by side-by-side overlap of atomic orbitals; the electron density is found on opposite sides of the internuclear axis
- sigma bond (σ bond)
- covalent bond formed by overlap of atomic orbitals along the internuclear axis
Chemistry End of Section Exercises
- Explain how σ and π bonds are similar and how they are different.
- Explain why bonds occur at specific average bond distances instead of the atoms approaching each other infinitely close.
- How many σ and π bonds are present in the molecule HCN?
- Draw the Lewis structures for CO2 and CO, and predict the number of σ and π bonds for each molecule.
- Do you agree with the following statement? Why or why not?
“N2 has three π bonds due to overlap of the three p-orbitals on each N atom.” - The bond energy of a C–C single bond averages 356 kJ mol−1; that of a C≡C triple bond averages 813 kJ mol−1. Explain why the triple bond is not three times as strong as a single bond.
- For the molecule allene, H2C=C=CH2, give the hybridization of each carbon atom. Will the hydrogen atoms be in the same plane or perpendicular planes?
- A useful solvent that will dissolve salts as well as organic compounds is the compound acetonitrile, H3CCN. It is present in paint strippers.
- Write the Lewis structure for acetonitrile, and indicate the direction of the dipole moment in the molecule.
- Identify the hybrid orbitals used by the carbon atoms in the molecule to form σ bonds.
- Describe the atomic orbitals that form the π bonds in the molecule.
- Describe the molecular geometry and hybridization of the N, P, or S atoms in each of the following compounds.
- H3PO4, phosphoric acid, used in cola soft drinks
- NH4NO3, ammonium nitrate, a fertilizer and explosive
- S2Cl2, disulfur dichloride, used in vulcanizing rubber
- K4[O3POPO3], potassium pyrophosphate, an ingredient in some toothpastes
- Identify the hybridization of the central atom in each of the following molecules and ions that contain multiple bonds:
- ClNO (N is the central atom)
- CS2
- Cl2CO (C is the central atom)
- Cl2SO (S is the central atom)
- SO2F2 (S is the central atom)
- ClOF2+ (Cl is the central atom)
- For each of the following structures, determine the hybridization requested and whether the π bond electrons are delocalized:
- Hybridization of each carbon
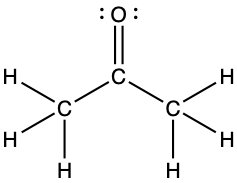
- Hybridization of sulfur

- All carbon and nitrogen atoms
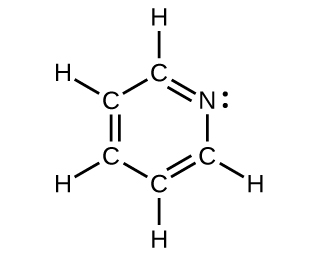
- Hybridization of each carbon
- For each of the following molecules, indicate the hybridization requested and whether or not the π bond electrons are delocalized:
- ozone (O3) central O hybridization
- carbon dioxide (CO2) central C hybridization
- nitrogen dioxide (NO2) central N hybridization
- phosphate ion (PO43−) central P hybridization
Answers to Chemistry End of Section Exercises
- Similarities: Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. Differences: σ bonds are stronger and result from end-to-end overlap and all single bonds are σ bonds; π bonds between the same two atoms are weaker because they result from side-by-side overlap, and multiple bonds contain one or more π bonds (in addition to a σ bond).
- The specific average bond distance is the distance with the lowest energy. At distances less than the bond distance, the positive charges on the two nuclei repel each other, and the overall energy increases.
- Two σ bonds and two π bonds.
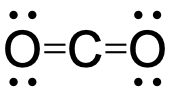 two σ bonds and two π bonds;
two σ bonds and two π bonds; 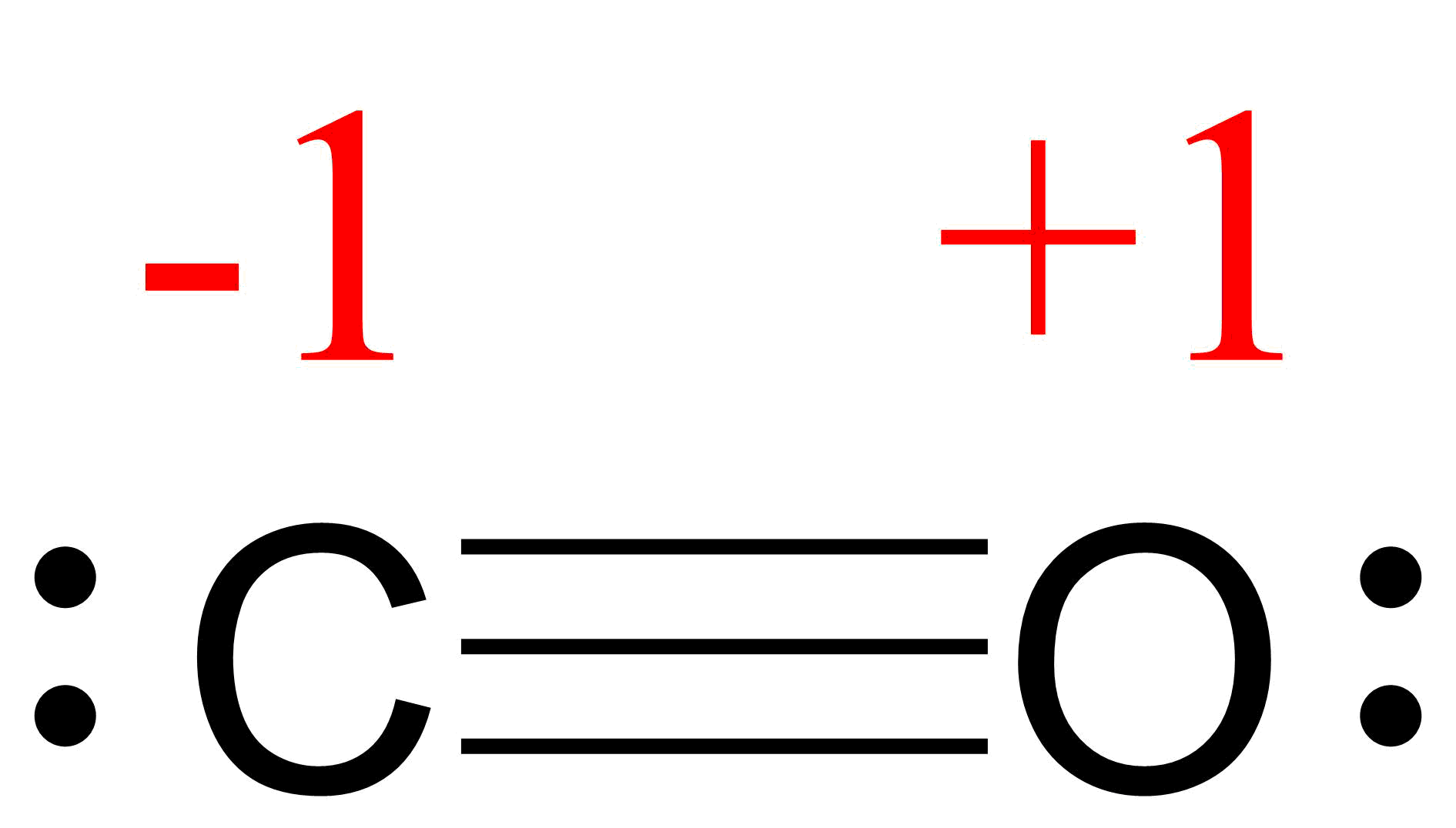 one σ bond and two π bonds
one σ bond and two π bonds- No, two of the p orbitals (one on each N) will be oriented end-to-end and will form a σ bond.
- A triple bond consists of one σ bond and two π bonds. A σ bond is stronger than a π bond due to greater overlap.
- From left to right: sp2, sp, sp2. The hydrogen atoms would be in perpendicular planes.
- (a)
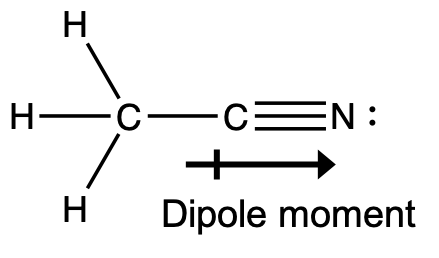
(b) The terminal carbon atom uses sp3 hybrid orbitals, while the central carbon atom is sp hybridized.
(c) Each of the two π bonds is formed by overlap of a 2p orbital on carbon and a nitrogen 2p orbital. - (a) P is sp3, tetrahedral; (b) the NH4 nitrogen is sp3, tetrahedral; the NO3 nitrogen is sp2, trigonal planar; (c) both S are sp3, bent; (d) both P are sp3, tetrahedral
- (a) sp2; (b) sp; (c) sp2; (d) sp3; (e) sp3; (f) sp3
- (a) from left to right, sp3, sp2, sp3, localized; (b) sp2, delocalized; (c) all are sp2, delocalized
- (a) sp2, delocalized; (b) sp, localized; (c) sp2, delocalized; (d) sp3, delocalized

