12 Acids, Bases, Neutralization, and Gas-Forming Reactions (M3Q3-4)
Introduction
Learning Objectives for Acid, Base, and Neutralization Reactions
- Define Brønsted acids and bases. Memorize the common strong and weak acids and bases.
| Acid-Base Reactions | - Describe the characteristics of a neutralization reaction.
| Neutralization Reactions | - Memorize and recognize the common gas-forming reactions.
| Acid Base Gas-Forming Reactions |
| Key Concepts and Summary | Glossary | End of Section Exercises |
Acid-Base Reactions
An acid-base reaction is one in which a hydrogen ion, H+, is transferred from one chemical species to another. Such reactions are of central importance to numerous natural and technological processes, ranging from the chemical transformations that take place within cells and the lakes and oceans, to the industrial-scale production of fertilizers, pharmaceuticals, and other substances essential to society. The subject of acid-base chemistry, therefore, is worthy of discussion.
For purposes of this brief introduction, we will consider only the more common types of acid-base reactions that take place in aqueous solutions. In this context, an acid is a substance that will dissolve in water to yield hydronium ions, H3O+. As an example, consider the equation shown here:
HCl(aq) + H2O(l) ⟶ Cl–(aq) + H3O+(aq)
The process represented by this equation confirms that hydrogen chloride (also commonly known as hydrochloric acid) is an acid. When dissolved in water, H3O+ ions are produced by a chemical reaction in which H+ ions are transferred from HCl molecules to H2O molecules (Figure 1b).
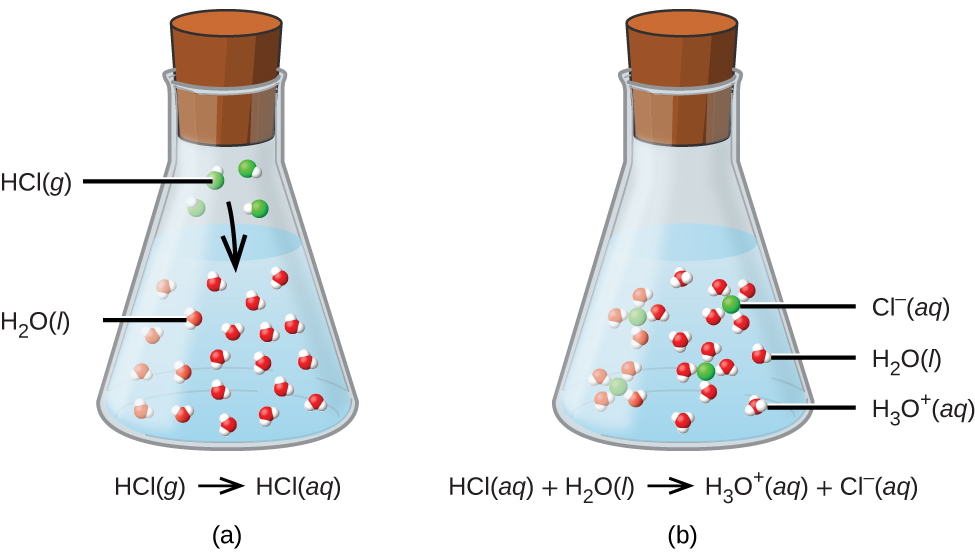
The nature of HCl is such that its reaction with water as just described is essentially 100% efficient: Virtually every HCl molecule that dissolves in water will undergo this reaction. Acids that completely react in this fashion are called strong acids, and HCl is one among just a handful of common acid compounds that are classified as strong (Table 1). Memorizing these strong acids is highly recommended! Even though a bottle of hydrochloric acid is labeled as HCl(aq), there are essentially no molecules of HCl present in solution due to the complete dissociation of the molecule to produce ions. HCl is therefore classified as a strong electrolyte (see the Dissolving and Electrolytes section of a previous section) and HCl(aq) is a conductive solution.
| Compound Formula | Name in Aqueous Solution | |
|---|---|---|
| HBr | hydrobromic acid | |
| HCl | hydrochloric acid | |
| HI | hydroiodic acid | |
| HNO3 | nitric acid | |
| HClO4 | perchloric acid | |
| H2SO4 | sulfuric acid | |
| Table 1. Common Strong Acids To Know | ||
A far greater number of compounds behave as weak acids and only partially react with water, leaving a large majority of dissolved molecules in their original form and generating a relatively small amount of hydronium ions. Weak acids are commonly encountered in nature, being the substances partly responsible for the tangy taste of citrus fruits, the stinging sensation of insect bites, and the unpleasant smells associated with body odor. A familiar example of a weak acid is acetic acid, the main ingredient in food vinegars:
CH3COOH(aq) + H2O(l) ⇌ CH3COO–(aq) + H3O+(aq)
When dissolved in water under typical conditions, only about 1% of acetic acid molecules are present in the ionized form, CH3COO–(aq) (Figure 2). (The use of a double-arrow in the equation above denotes the partial reaction aspect of this process.) Weak acids, as their name suggests, are classified as weak electrolytes due to the low concentration of ions in solution. To see how these conduct electricity, refer back to the conductivity movies presented in a previous section.
Not only is sulfuric acid, H2SO4, a strong acid, it is also a diprotic acid as it contains two protons, which is a common way to refer to H+ ions since H+ contains 1 proton and 0 electrons.. The dissociation of diprotic acids in water is best described using two separate equations with the first equation describing the transfer of one proton to water, and the second equation describing the transfer of the second proton from the HSO4–(aq) produced in the first equation:
H2SO4(aq) + H2O(l) ⟶ HSO4–(aq) + H3O+(aq) strong acid, fully dissociated
HSO4–(aq) + H2O(l) ⇌ SO42-(aq) + H3O+(aq) weak acid, partially dissociated
Example 1
Writing Dissociation equations for Acids in Water
Write the chemical equation that describes the dissociation of nitric acid, HNO3, in water.
Solution
HNO3 is a strong acid (Table 1) and therefore completely dissociates in water:
HNO3(aq) + H2O(l) ⟶ H3O+(aq) + NO3–(aq)
Write the chemical equation that describes the dissociation of perchloric acid in water.
Answer:
HClO4(aq) + H2O(l) ⟶ H3O+(aq) + ClO4–(aq)
Example 2
Writing Dissociation equations for Acids in Water
Write the chemical equation that describes the dissociation of nitrous acid, HNO2, in water.
Solution
HNO2 is not in Table 1 and is therefore a weak acid and will only partially dissociate in water, hence the double arrow in the equation below.
HNO2(aq) + H2O(l) ⇌ H3O+(aq) + NO2–(aq)
Write the chemical equation that describes the dissociation of hypochlorous acid, HClO(aq), acid in water.
Answer:
HClO(aq) + H2O(l) ⇌ H3O+(aq) + ClO–(aq)
A base is a substance that will dissolve in water to yield hydroxide ions, OH−. The most common bases are ionic compounds composed of alkali or alkaline earth metal cations (groups 1 and 2) combined with the hydroxide ion—for example, NaOH and Ca(OH)2. When these compounds dissolve in water, hydroxide ions are released directly into the solution. For example, KOH and Ba(OH)2 dissolve in water and dissociate completely to produce cations (K+ and Ba2+, respectively) and hydroxide ions, OH−. These bases, along with other hydroxides that completely dissociate in water, are considered strong bases.
| Compound Formula | Name in Aqueous Solution | |
|---|---|---|
| LiOH | lithium hydroxide | |
| NaOH | sodium hydroxide | |
| KOH | potassium hydroxide | |
| Ca(OH)2 | calcium hydroxide | |
| Sr(OH)2 | strontium hydroxide | |
| Ba(OH)2 | barium hydroxide | |
| Table 2. Common Strong Bases | ||
Consider as an example the dissolution of lye (sodium hydroxide) in water:
NaOH(s) ⟶ Na+(aq) + OH–(aq)
This equation confirms that sodium hydroxide is a base. When dissolved in water, NaOH dissociates to yield Na+ and OH− ions. This is also true for any other ionic compound containing hydroxide ions. Since the dissociation process is essentially complete when ionic compounds dissolve in water under typical conditions, NaOH and other ionic hydroxides are all classified as strong bases.
Unlike ionic hydroxides, some compounds produce hydroxide ions when dissolved by chemically reacting with water molecules. In all cases, these compounds react only partially and are therefore classified as weak bases. These types of compounds are also abundant in nature and important commodities in various technologies. For example, global production of the weak base ammonia is typically well over 100 metric tons annually, being widely used as an agricultural fertilizer, a raw material for chemical synthesis of other compounds, and an active ingredient in household cleaners (Figure 3). When dissolved in water, ammonia reacts partially to yield hydroxide ions, as shown here:
NH3(aq) + H2O(l) ⇌ NH4+(aq) + OH–(aq)
This is, by definition, an acid-base reaction, in this case involving the transfer of H+ ions from water molecules to ammonia molecules. Under typical conditions, only about 1% of the dissolved ammonia is present as NH4+ ions, and NH3(aq) is a weak electrolyte.

Neutralization Reactions
Reactions between strong acids and bases
The chemical reactions described in which acids and bases dissolved in water produce hydronium and hydroxide ions, respectively, are, by definition, acid-base reactions. In these reactions, water serves as both a solvent and a reactant. A neutralization reaction is a specific type of acid-base reaction in which the reactants are an acid and a base, the products are often a salt and water, and neither reactant is the water itself:
To illustrate a neutralization reaction, which are another category of double displacement reactions, consider what happens when a typical antacid such as milk of magnesia (an aqueous suspension of solid Mg(OH)2) is ingested to ease symptoms associated with excess stomach acid (HCl):
Mg(OH)2(s) + 2 HCl(aq) ⟶ MgCl2(aq) + 2 H2O(l)
Note that in addition to water, this reaction produces a salt, magnesium chloride. When writing these equations it is essential that you recognize the ions involved in the chemical reaction and their appropriate charges to ensure the correct formula for the products is written before any attempt is made to balance the equation.
Here, the magnesium hydroxide is solid, so it must be written as Mg(OH)2(s). The complete ionic equation for this reaction is:
Mg(OH)2(s) + 2 H+(aq) + 2 Cl–(aq) ⟶ Mg2+(aq) + 2 Cl–(aq) + 2 H2O(l)
Canceling out the spectator Cl–(aq) ions gives the net ionic equation:
Mg(OH)2(s) + 2 H+(aq) ⟶ Mg2+(aq) + 2 H2O(l)
Demonstration: Conductivity titration of a strong base with a strong acid
Set up. This demonstration shows the titration of a strong base, Ba(OH)2, with a strong acid (H2SO4). The buret contains 0.1 M H2SO4 and the dish contains 0.1 M Ba(OH)2. Phenolphthalein indicator is also added to the dish. This indicator, as we saw in a previous demo, is colorless when the solution is acidic and pink when the solution is basic. Before watching the video, write the reaction equation and predict whether the solution will transition from colorless to pink, or from pink to colorless.
In this demonstration, we are also observing the conductivity using a similar set-up to what we used in the electrolyte demonstration earlier in this module. Remember that electricity will flow (and light the bulb) when there are electrolytes in solution.
Prediction. Before watching the video, write the reaction equation for this experiment and form a hypothesis about if and when the bulb will be lit.
Explanation. The reaction occurring in this demonstration is:
H2SO4(aq) + Ba(OH)2(aq) ⟶ BaSO4(s) + 2 H2O(l)
This video shows that the strong base, Ba(OH)2, is a strong electrolyte and conducts electricity in order to light the bulb. This is in agreement with what we saw in our earlier electrolyte demonstration. As H2SO4 is added, the solution fades from pink to colorless and forms solid barium sulfate. The light bulb begins to dim as the equivalence point is approached. The equivalence point is the point at which all of the base in the dish is completely neutralized by the addition of the strong acid. At this point, all ions are neutralized and are present as a solid salt and therefore there are no more mobile ions in solution to carry the charge. The bulb is off. When more H2SO4 is added after the equivalence point, there are excess H+ and SO42- ions in solution to carry the charge, and the bulb lights up again.
Reactions involving weak acids and bases
Neutralization reactions between weak acids and strong bases also produce a salt and water as products, but care must be taken when writing the complete and net ionic equations as weak acids are only slightly dissociated in solution.
To illustrate, consider the reaction between weak acid HNO2(aq) and strong base NaOH(aq) described below:
HNO2(aq) + NaOH(aq) ⟶ NaNO2(aq) + H2O(l) overall equation
Because HNO2(aq) is only partially dissociated and it exists mainly in its molecular form in solution, it is not logical to write it as separate ions in the complete ionic equation. It is therefore unchanged in the ionic equations. However, NaOH(aq) is a strong base and should be represented as separate ions, as should NaNO2, which is a soluble salt.
HNO2(aq) + Na+(aq) + OH–(aq) ⟶ Na+(aq) + NO2–(aq) + H2O(l) complete ionic equation
Canceling out the Na+(aq) spectator ions produces the net ionic equation:
HNO2(aq) + OH–(aq) ⟶ NO2–(aq) + H2O(l) net ionic equation
Example 3
Writing Equations for Acid-Base Reactions
Write balanced chemical equations (overall, complete ionic and net ionic) for the reaction when a solution of barium hydroxide is neutralized with a solution of nitric acid.
Solution
The two reactants are Ba(OH)2 and HNO3. Since this is a neutralization reaction between a strong acid and strong base, the two products will be water and a salt composed of the cation of the ionic hydroxide (Ba2+) and the anion generated when the acid transfers its hydrogen ion (NO3−).
Write the overall, complete ionic and net ionic equation representing the neutralization of solid calcium hydroxide and perchloric acid.
Answer:
Ca(OH)2(s) + 2 HClO4(aq) ⟶ Ca(ClO4)2(aq) + 2 H2O(l) overall equation
Ca(OH)2(s) + 2 H+(aq) + 2 ClO4–(aq) ⟶ Ca2+(aq) + 2 ClO4–(aq) + 2 H2O(l) complete ionic equation
Ca(OH)2(s) + 2 H+(aq) ⟶ Ca2+(aq) + 2 H2O(l) net ionic equation
Check Your Learning
Write the overall, complete ionic, and net ionic equation that describes the reaction between the weak acid CH3COOH(aq) and KOH(aq).
Answer:
CH3COOH(aq) + KOH(aq) ⟶ CH3COOK(aq) + H2O(l) overall equation
CH3COOH(aq) + K+(aq) + OH–(aq) ⟶ CH3COO–(aq) + K+(aq) + H2O(l) complete ionic equation
CH3COOH(aq) + OH–(aq) ⟶ CH3COO–(aq) + H2O(l) net ionic equation

Explore the microscopic view of strong and weak acids and bases.
Acid Base Gas-Forming Reactions
Ionic compounds containing carbonate, sulfite, and sulfide anions are bases that react with acids to produce a salt, water, and a gas. Carbonates produce gaseous carbon dioxide, metal sulfites produce gaseous sulfur dioxide, and metal sulfides produce gaseous hydrogen sulfide as outlined in the ionic reaction schemes below:
CO32-(aq) + 2 H+(aq) → H2CO3 (aq, Unstable) → CO2(g) + H2O(l)
SO32-(aq) + 2 H+(aq) → H2SO3 (aq, Unstable) → SO2(g) + H2O(l)
S2-(aq) + 2 H+(aq) → H2S(g)
The H2CO3 (carbonic acid) and H2SO3 (sulfurous acid) formed when carbonates and sulfites react with acids are generally unstable when formed in solution in an open container and immediately decompose to form the respective gas and water. For example, when hydrochloric acid is added to solid calcium carbonate, bubbles of carbon dioxide are immediately observed. This chemical reaction is described by the following overall, complete ionic, and net ionic equations:
CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l) overall equation
CaCO3(s) + 2 H+(aq) + 2 Cl–(aq) → Ca2+(aq) + 2 Cl–(aq) + CO2(g) + H2O(l) complete ionic equation
CaCO3(s) + 2 H+(aq) → Ca2+(aq) + CO2(g) + H2O(l) net ionic equation
The reaction of calcium sulfite with hydrochloric acid is very similar to the above reaction, the overall equation given below:
CaSO3(s) + 2 HCl(aq) → CaCl2(aq) + SO2(g) + H2O(l) overall equation
The overall equation describing the reaction between sodium sulfide and hydrochloric acid is written below:
Na2S(s) + 2 HCl(aq) → 2 NaCl(aq) + H2S(g)
Key Concepts and Summary
Chemical reactions are classified according to similar patterns of behavior. This section discussed basic properties of acids, bases, neutralization reactions, and gas-forming reactions. Acids produce H+ ions in solution, while bases produce OH– ions in solution. Neutralization reactions occur between acids and bases and produce a salt and water. The term “salt” is used to refer to any ionic compound. When writing complete and net ionic equations for neutralization reactions between a strong base and weak acid or between a weak base and strong acid, it is important to remember that weak acids and bases do not dissociate to a large extent in solution. Weak acids and bases remain intact in a complete and net ionic equation.
Ionic compounds containing carbonate (CO32-), sulfite (SO32-), and sulfide (S2-) are bases that form salt, water and a gas when reacted with acids.
Glossary
Chemistry End of Section Exercises
- Complete and balance the following acid-base equations:
- HCl gas reacts with solid Ca(OH)2.
- A solution of Sr(OH)2 is added to a solution of HNO3.
- Complete and balance the following acid-base equations:
- A solution of HClO4 is added to a solution of LiOH.
- Aqueous H2SO4 reacts with NaOH.
- Ba(OH)2 reacts with HF gas.
- Complete and balance the equations for the following acid-base neutralization reaction. If water is used as a solvent, write the reactants and products as aqueous ions. In some cases, there may be more than one correct answer, depending on the amounts of reactants used.
- Mg(OH)2(s) + HClO4(aq) →
Answers to Chemistry End of Section Exercises
- (a) 2HCl(g) + Ca(OH)2(s) → CaCl2(s) + 2 H2O(l)
(b) Sr(OH)2(aq) + 2 HNO3(aq) → Sr(NO3)2(aq) + 2 H2O(l) - (a) HClO4(aq) + LiOH(aq) → LiClO4(aq) + H2O(l)
(b) H2SO4(aq) + 2 NaOH(aq) → Na2SO4(aq) + 2 H2O(l)
(c) Ba(OH)2(aq) + 2 HF(g) → BaF2(s) + 2 H2O (l) - Mg(OH)2(s) + 2 HClO4(aq) → Mg2+(aq) + 2 ClO4–(aq) + 2 H2O(l)

