Unit Four
Day 29: Gibbs Free Energy, Chemical Equilibrium
D29.1 Temperature Dependence of Gibbs Free Energy
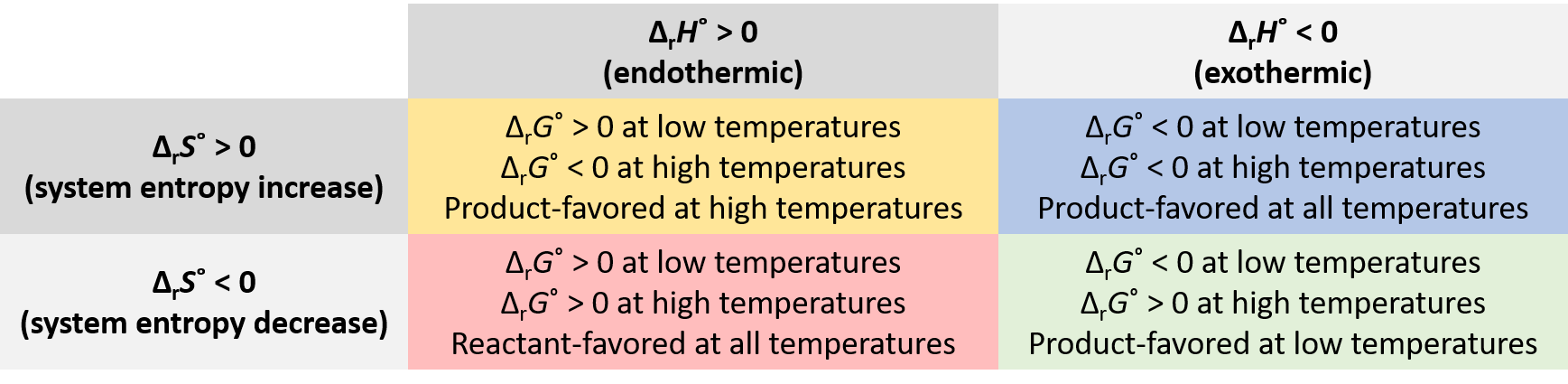
Whether a reaction is product-favored, that is, whether the reactants are converted to products under standard-state conditions, is reflected in the arithmetic sign of its ΔrG°. This equation
shows that the sign of ΔrG° depends on the signs of ΔrH° and ΔrS°, and, in some cases, the absolute temperature (which can only have positive values). Four possibilities exist:
- Both ΔrH° and ΔrS° are positive—an endothermic process with an increase in system entropy. ΔrG° is negative if TΔrS° > ΔrH°, and positive if TΔrS° < ΔrH°. Such a process is product-favored at high temperatures and reactant-favored at low temperatures.
- Both ΔrH° and ΔrS° are negative—an exothermic process with a decrease in system entropy. ΔrG° is negative if |TΔrS°| < |ΔrH°| and positive if |TΔrS°| > |ΔrH°|. Such a process is product-favored at low temperatures and reactant-favored at high temperatures. (Remember that |TΔrS°| represents the magnitude of TΔrS°, ignoring mathematical sign.)
- ΔrH° is positive and ΔrS° is negative—an endothermic process that with a decrease in system entropy. ΔrG° is positive regardless of the temperature. Such a process is reactant-favored at all temperatures.
- ΔrH° is negative and ΔrS° is positive—an exothermic process with an increase in system entropy. ΔrG° is negative regardless of the temperature. Such a process is product-favored at all temperatures.
These four scenarios are summarized in Figure 1.
Activity 1: Temperature and Product-favored or Reactant-favored Reactions
Figure 2 illustrates the four scenarios graphically, where ΔrG° is plotted versus temperature:
| ΔrG° | = | − ΔrS°(T) | + | ΔrH° |
| y | = | m(x) | + | b |
For most reactions, neither ΔrH° nor ΔrS° change significantly as temperature changes. Thus, in Figure 2, the lines representing ΔrG° are linear because the slope of each line (−ΔrS°) is the same at all temperatures. The orange and green plots (representing examples of scenario 1 and 2, respectively) cross from product-favored to reactant-favored (as reflected by the sign of ΔrG°) at a temperature that is characteristic of the specific process. This temperature is represented by the x-intercept, the value of T for which ΔrG° is zero:
Hence, saying a process is product-favored at “high” or “low” temperatures is simply indicating whether the temperature is above or below TΔrG°=0. These relative terms are reaction-specific, that is, what is a "high" temperature for one reaction may very well be a "low" temperature for another reaction.
Exercise 1: Estimating Boiling Point
D29.2 Chemical Equilibrium
A chemical reaction is usually written with a single arrow, which suggests it proceeds in one direction, the direction of the arrow. But all chemical reactions are reversible, and both the forward and reverse reaction occur simultaneously. When reactions involve gases or solutions, where concentrations change as the reaction proceeds, the reaction eventually reaches a dynamic chemical equilibrium.
In a chemical equilibrium, the forward and reverse reactions occur at the same rates, and the concentrations of products and reactants remain constant over time. This implies that, if a reaction occurs in a closed system so that the products cannot escape, the reaction often does not yield 100% products. Instead, some reactants remain after the concentrations stop changing. At this point, when there is no further change in concentrations of reactants and products, we say the reaction is at equilibrium.
For example, when we place a sample of dinitrogen tetraoxide (N2O4, a colorless gas) in a glass vessel, the color becomes darker as N2O4 is converted to nitrogen dioxide (NO2, a red-brown gas) by the reaction:
| N2O4(g) | 2NO2(g) |
Activity 2: Reaction Energy Diagram
At the beginning of this reaction, there is pure N2O4. As soon as the forward reaction produces some NO2, at rate = kf[N2O4]t, the reverse reaction begins to occur at rate = kr[NO2]t2, and NO2 starts to react to form N2O4. (The subscripts, t, indicate a time before equilibrium is reached.) As the reaction proceeds, the rate of the forward reaction decreases as [N2O4]t decreases and the rate of the reverse reaction increases as [NO2]t increases. When the system reaches equilibrium, both N2O4 and NO2 are present.
At equilibrium, [N2O4] and [NO2] no longer change over time because the rate of NO2 formation is exactly equal to the rate of NO2 consumption, and the rate of N2O4 formation is exactly equal to the rate of N2O4 consumption. Chemical equilibrium is a dynamic process: the numbers of reactant and product molecules remain constant, but the forward and reverse reactions do not stop.
We use the ⇌ arrow when writing an equation for a reversible reaction. Such a reaction may or may not be at equilibrium. When we wish to speak about one particular aspect of a reversible reaction, we use a single arrow. For example, when the reaction in Figure 3 is at equilibrium, the rate of the forward reaction:
is equal to the rate of the reverse reaction:
An equilibrium can be established for a physical change as well as for a chemical reaction. For example:
Figure 4 shows a sample of liquid Br2 at equilibrium with Br2 vapor in a closed container. When we pour liquid Br2 into an empty bottle in which there is no bromine vapor, some liquid evaporates: the amount of liquid decreases and the amount of vapor increases. If we seal the container so no vapor escapes, the amount of liquid and vapor will eventually stop changing; at that point an equilibrium between the liquid and the vapor has been established. If the container were not sealed, the bromine vapor would escape and no equilibrium would be reached.

D29.3 Concentration Equilibrium Constants
A concentration equilibrium constant (Kc ) is a ratio of equilibrium concentrations of products and reactants that is constant for a given reaction at a given temperature. For example, consider this generic reversible reaction:
For this reaction, the concentration equilibrium constant, Kc, is:
This mathematical expression is called the equilibrium constant expression. The "[...]e" expression indicates explicitly equilibrium concentration of a reactant or product.
As a reaction approaches equilibrium, concentrations of reactants and products need to change until the rates of forward and reverse reactions are equal. Therefore, only substances whose concentrations can change as a reaction occurs are included in an equilibrium constant expression. For example, consider the following reaction at equilibrium:
2 HgO(s) ⇌ 2 Hg(l) + O2(g) Kc = [O2]e
Because HgO is a pure solid, the number of HgO formula units in a given volume of HgO is the same throughout the reaction; it depends only on the density of HgO at the temperature of the reaction. Similarly, the number of Hg atoms per unit volume of pure Hg(l) is constant throughout the reaction. Thus, these concentrations are not included in the Kc expression. It is necessary for some HgO(s) and some Hg(l) to be present for the equilibrium to be maintained, but the quantity of each does not matter.
In general, Kc expressions do not contain terms for pure solids or pure liquids. In addition, for dilute solutions, the concentration of solvent remains constant throughout an equilibrium reaction and is also not included in the Kc expression, even though the solvent may appear in the reaction equation.
A homogeneous equilibrium is one in which all of the reactants and products are present in a single phase. Examples of homogeneous equilibria are reactions in the gas phase and reactions in liquid solutions. For example:
In the aqueous equilibrium systems, H2O(l) is the solvent. Its concentration does not appear in the Kc expression.
A heterogeneous equilibrium is a system in which reactants and products are found in two or more phases. Some heterogeneous equilibria involve chemical changes, for example:
Other heterogeneous equilibria involve phase changes, for example:
Working with Kc
When all the coefficients in a balanced chemical equation are multiplied by some factor n, then the new Kc is the original Kc raised to the nth power. For example:
When a reaction’s direction is reversed, the Kc for the new reaction is the reciprocal (inverse) of the original reaction Kc. For example:
When two reactions occur sequentially to yield a new overall reaction, the Kc for the overall reaction is the product of the Kc values for the individual steps. For example:
Exercise 2: Properties of Chemical Equilibrium
D29.4 Equilibrium Constant and Partial Pressure
Reactions in which all reactants and products are in the gas phase are another class of homogeneous equilibria. In these cases, the partial pressure of each gas can be used instead of its concentration in the equilibrium constant equation. At constant temperature, the partial pressure of a gas is directly proportional to its molar concentration (c), which is the amount of a substance (moles) per unit volume (liters): c = n/V. This proportionality of pressure and concentration can be derived from the ideal gas equation:
For an example, consider the following reaction:
We can write the equilibrium constant, Kp, using the equilibrium partial pressures of the gases, by following the same guidelines as for Kc expressions:
The two equilibrium constants, Kc and Kp, are directly related to each other. For the generic gas-phase reaction:
where Δn is the difference between the sum of the coefficients of the gaseous products and the sum of the coefficients of the gaseous reactants in the reaction (that is, the change in amount of gas between the reactants and the products).
Note that the gas constant, R, can be expressed in different units. Use the R value and associated units that match the partial pressure units used in the Kp expression.
For heterogeneous equilibria that involve gases, equilibrium constants can also be expressed using partial pressures instead of concentrations. Two examples are:
Exercise 3: Pressure and Concentration Equilibrium Constant Expressions
Exercise 4: Converting Pressure and Concentration Equilibrium Constants
D29.5 Calculations Involving Equilibrium Constants
One way to determine the value for an equilibrium constant is to measure the concentrations (or partial pressures) of all reactants and all products at equilibrium.
Exercise 5: Calculating an Equilibrium Constant
Calculate the equilibrium constant Kc for the decomposition of PCl5 at 250 °C.
At equilibrium, [PCl5]e = 4.2 × 10-5 M, [PCl3]e = 1.3 × 10-2 M, [Cl2]e = 3.9 × 10-3 M
A known equilibrium constant can be used to calculate an unknown equilibrium concentration, provided the equilibrium concentrations of all other reactants and products are known.
Exercise 6: Equilibrium Constant and Air Pollution
D29.6 Equilibrium Constants and Product-favored Reactions
The magnitude of an equilibrium constant is a measure of the yield of a reaction when it reaches equilibrium. A very large value for Kc (Kc >> 1) indicates that product concentrations are much larger than reactant concentrations when equilibrium has been achieved: nearly all reactants have been converted into products. If Kc is large enough, the reaction has gone essentially to completion when it reaches equilibrium.
Earlier we defined a product-favored reaction as one that proceeds spontaneously in the forward direction when all concentrations (or partial pressures) have the standard-state value of 1 M. If Kc > 1,so that the concentrations of products are greater than the concentrations of reactants, then when all concentrations are 1 M the reaction needs to produce greater concentrations of products to reach equilibrium. That is, the reaction needs to proceed in the forward direction. Thus, Kc > 1 (or Kp > 1) means that a reaction is product-favored.
A very small value of Kc, (Kc << 1) indicates that equilibrium is achieved when only a small fraction of the reactants has been converted into products. Such a reaction is reactant-favored. If Kc is small enough, essentially no reaction has occurred when equilibrium is reached. When Kc ≈ 1, both reactant and product concentrations are significant and it is necessary to use the equilibrium constant to calculate equilibrium concentrations.
By an argument similar to the one in the next to last paragraph above, Kc < 1 (or Kp < 1) means that a reaction is reactant-favored.
Exercise 7: Identifying Reactant-Favored and Product-Favored Processes
Podia Question
One way to remove silver oxide tarnish from silver is to heat the silver to a high temperature.
The reaction is:
2 Ag2O → 4 Ag(s) + O2(g)
At 298 K, the reaction is reactant-favored. Obtain data from the Appendix and determine at what temperature the reaction becomes product-favored. What assumptions need to be made to solve this problem?
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

