Unit Two
Day 11: Molecular Structure: Isomers
D11.1 Line Structures
Think about the importance of structures of covalent molecular substances. Hydrocarbons (Section D7.2) or closely related molecules are good examples because there are many different hydrocarbon molecules and many hydrocarbons have lots of atoms. Knowing which atoms are bonded to which and the different ways they are bonded enables predictions about the properties of the corresponding compounds.
For example, molecules of hydrocarbons in motor oil for automobiles contain from 16 to 20 carbon atoms and more than twice as many associated hydrogen atoms. Drawing Lewis structures for such large molecules takes time and can be overly complicated, so a simpler kind of drawing, called a line structure, is often used. In a line structure (also called a skeletal structure) for a hydrocarbon, only the C–C bonds are shown; element symbols and C–H bonds are omitted. Carbon atoms are represented by the end of a line or a juncture between two lines. Figure 1 shows an example line structure. Decide where each C atom and each H atom is in the line structure; then count the number of C and H atoms to verify that the line structure represents the same molecule as the Lewis structure.
Line structures are much easier to draw, and the general molecular structure of the carbon chain or chains is clearer. However, it is also much easier to make mistakes when drawing and reading line structures: an extra line is a whole extra CHn group and it is also easier to miss some of the implied C-H bonds.
Exercise 1: Interpreting Line Structures
In line structures, atoms other than carbon and hydrogen are represented by their elemental symbols, and any H atoms bonded to them are shown explicitly (for example, see molecule C in the exercise above). In line structures, lone pairs on atoms are often omitted as well. Because C-H bonds and lone pairs are not shown, it is necessary to include the formal charge on any atom that has a nonzero formal charge when drawing a line structure. Otherwise, there would be a miscount of bonds or electrons.
Exercise 2: Degree of Unsaturation and Line Structures
D11.2 Isomeric Structures
When more than one molecular structure corresponds to the same molecular formula the two or more structures are called isomeric structures, or isomers. For example, there are two structures corresponding to the formula CHN. They are H–C≡N: (hydrogen cyanide) and H–N≡C: (hydrogen isocyanide). At room temperature and especially at lower temperatures, both structures exist as separate compounds. That is, the substance represented by one structure can be purified and separated from the other and each substance has different properties.
When molecular formulas are different, the formulas must describe substances with different properties because the formula tells how many atoms of each kind are in a molecule and chemical bonds would have to be broken to change the number or atoms or type of atoms. Breaking covalent bonds requires energy, and at room temperature very few molecules have enough energy for bond breaking to occur. Isomers occur when two different molecular structures with the same number of atoms of each type do not have sufficient energy to change from one structure to the other. For example, H–N≡C: does not change into H–C≡N: because the change requires breaking a N–H bond. At room temperature the H–N≡C: molecules do not have enough energy to overcome the bond enthalpy of the N–H bond, so they do not change; thus, H–N≡C: can be purified and separated from H–C≡N:.
Typically isomers are defined by whether they can interchange at room temperature. At room temperature molecules do not have sufficient energy to break most covalent bonds. Thus, if interchanging two structures requires breaking a covalent bond, the two structures are isomers. If the temperature is raised, the molecules have greater average energy and it becomes more likely that one structure can change into another. If the temperature is lowered, it becomes harder to interchange structures; that is, the average energy of the molecules is lower and structures that could interchange at room temperature no longer have sufficient energy.
D11.3 Conformations
At room temperature, molecules are in constant motion both with respect to other molecules and as a result of internal motions such as rotation of one part of a molecule relative to another part of the same molecule. Below is an animation of a butane molecule at room temperature. Notice that there is rotation around the central C–C single bond (which is kept stationary in the animation to show the rotation better) and also around the other C–C bonds. Such rotations in a molecule lead to different conformations or conformers, structures that differ only because of rotations around single bonds.
Activity 1: Depicting Conformers
Rotations around C–C bonds require little energy because the molecular orbital of a σ covalent bond has cylindrical symmetry along the internuclear axis (Section D5.5, Section D10.1). Cylindrical symmetry means that regardless of how two σ-bonded atoms are rotated with respect to each other around the internuclear axis, the σ bond remains unbroken between them. For example, consider the rotation around the C-C bond in 1,2-dichloroethane:
Notice in Figure 3 that complete rotation around the C–C bond requires an energy change of 40 kJ/mol. The bond enthalpy of a C–C bond is 346 kJ/mol, so the energy required for rotation is a bit more than one-tenth the energy required to break the bond. Because the energy required for rotation around single bonds is small, such rotations occur readily at room temperature for most molecules. Hence, at room temperature, one conformer cannot be isolated from another and chemists consider that conformers represent the same chemical compound, with the same name and the same physical properties. No bond breaking is needed to go from one conformer to another.
If you could see all molecular structures in a room-temperature sample of 1,2-dichloroethane at a specific instant, you would find all three of these structures:
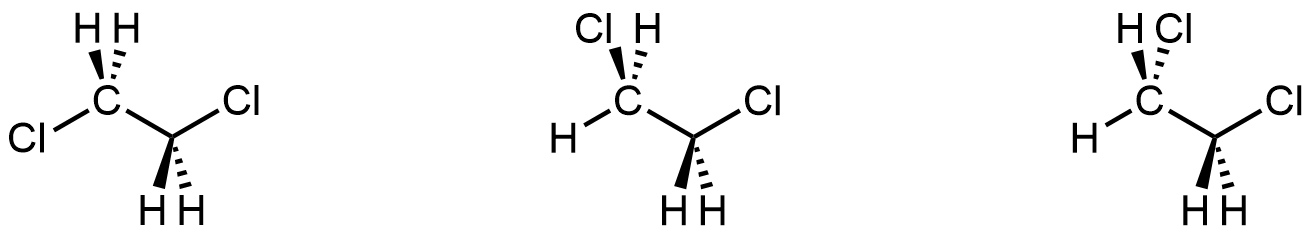
Each structure corresponds to one of the minima in the energy curve in Figure 3. If, instead, you followed one molecule over time, you would see it go from one structure to the next as the C-C bond rotates (as seen in Figure 3). Because experiments are typically done at room temperature, we consider all three structures as conformers of the same molecule. You can draw any of them as a representation of 1,2-dichlororethane.
From this animation of a nonane molecule you can see that different conformers of the same molecule can adopt, at a first glance, dramatically different molecular shapes. If you change your viewpoint by moving the molecule with your mouse, you can see that the molecule also looks quite different from different perspectives.
Given two or more Lewis structures, it is important that you can recognize whether they are conformers or isomers. Lewis structures or line structures that look quite different may be different conformers or the same conformer drawn from different perspectives; in either case, the structures represent the same substance.
A good way to tell whether two structures represent the same substance is to work out the correct name of each structure. If the names are the same, the structures represent conformers, not different substances. You will not be explicitly tested on naming compounds (nomenclature) in this course, but we strongly recommend that you study the section about alkanes in the appendix IUPAC nomenclature. IUPAC names are a systematic way of naming chemical compounds that can also help you when thinking about molecular structure. Going forward, if you encounter a name for a chemical compound that’s unfamiliar to you, the IUPAC nomenclature appendix can help.
Activity 2 Identifying Conformers
Chemically, different conformers can affect the reactivity of a molecule. And on a larger scale, huge protein molecules adopt only a few of many possible conformations; the shapes of protein molecules are essential to their biological functions.
D11.4 Constitutional Isomers
Compounds with the same molecular formula but different atomic connectivity are called constitutional isomers (or structural isomers). For example, there are two alkanes with the formula C4H10:
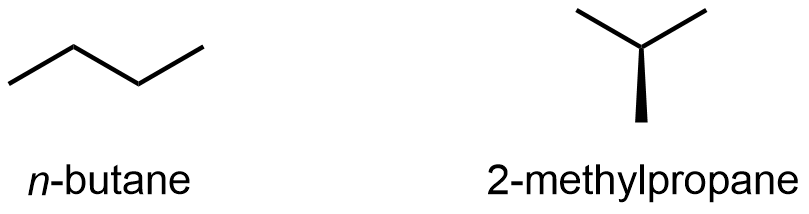
The n– in n-butane stands for normal, meaning an unbranched carbon chain. Typically the “n-” is omitted and the compound is just named “butane”; it is assumed that you know that no prefix refers to the unbranched chain.
The traditional name of 2-methylpropane is isobutane. Many times, the “2-” is omitted and the compound is just called “methylpropane” because that’s the only location for the methyl group to bond to (if the methyl is bonded to either end, the molecule is n-butane); hence there’s no ambiguity by omitting the “2-“.
Activity 3 Analyzing Constitutional Isomers
In your course notebook write an answer to each question and an explanation of your answer:
- Describe the shape of the n-butane molecule and how it differs from the shape of the 2-methylpropane molecule.
- Analyze the bonding in each molecule. Are all C atoms bonded to the same number of H atoms? If not, is the number of similarly bonded C atoms the same in each of the two structures ?
- To convert from n-butane to 2-methylpropane, would one or more chemical bonds need to be broken so that atoms could be re-arranged?
- Would you expect the physical properties (such as melting point and boiling point) to be different for the two substances?
To change from one constitutional isomer to another requires breaking and re-forming chemical bonds, which requires significant input of energy. At room temperature, very few molecules have that much energy. Therefore, constitutional isomers can be synthesized and separated from one another: they are different substances.
In addition to examining the connectivity in Lewis structures, another way to tell whether a Lewis structure represents the same molecule as another Lewis structure is to work out the IUPAC names for both structures. IUPAC names are designed so that the same structure always has the same name; if two structures are different, naming them correctly will result in different names.
Exercise 3: Naming Alkanes
Alkenes and alkynes can also exhibit constitutional isomerism. For example, the four carbon atoms in the chain of butene give rise to three different constitutional isomers:
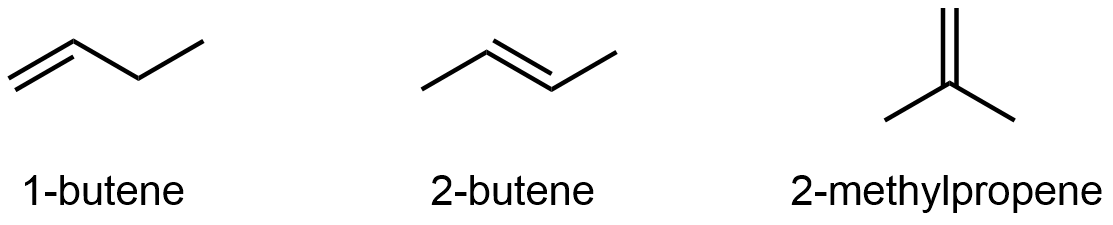
It’s not just the π bond location that differs, be sure that you can deduce all the C-H and C-C bonds that need to be broken and re-formed going from one isomer to the another by looking at just the line structures. See the 3D models of 1-butene, 2-butene, and 2-methylpropene to verify.
And similarly, butyne can have two different constitutional isomers:

Exercise 4: Isomers of Substituted Benzenes
The structure of ortho-xylene is shown here:
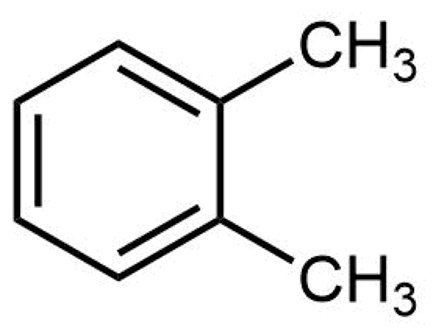
Click on each structure below that is a constitutional isomer of ortho-xylene.
D11.5 Stereoisomers: Geometric Isomers
Stereoisomers are molecules that have the same molecular formula and the same atomic connectivity, but differ in the orientation of the atoms in 3D space. Hence, molecules that are stereoisomers of each other stem from the same structural isomer. There are several different types of stereoisomers. First we will discuss the geometric isomers.
The two carbon atoms in a C=C double bond cannot freely rotate with respect to each other because such rotation requires breaking the π bond (see Section D10.4). Once the π bond is broken there would be free rotation around the remaining σ bond, but at room temperature most molecules do not have sufficient energy to break a π bond. Thus, there is no free rotation around a C=C double bond. This gives rise to geometric isomers, stereoisomers that differ in the orientation of the groups connected to a C=C bond.
For example, there are two geometric isomers of 2-butene, cis-2-butene and trans-2-butene:

The isomer with both methyl groups on the same side of the double bond is called a cis isomer (above, the methyl groups are shown as both being above the double bond). The one with the methyl groups on opposite sides is called a trans isomer. Geometric isomers have different physical properties, such as boiling point, that make separating them possible. Hence they are different substances, and in nomenclature, they are distinguished by the “cis” and “trans” prefix. Figure 5 shows an animation of a cis-2-butene molecule showing conformations that are accessible at room temperature.
Cis-trans isomerism is only possible when there are two different groups at each end of a double bond. For example, in an alkene molecule like this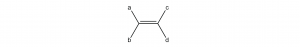
there can be geometric isomers if a and b are different groups and c and d are different groups. If both groups on either end of the double bond were the same (a the same as b, or c the same as d), rotating around the double bond would not produce a different molecular structure.
Exercise 5: Isomers
D11.6 Stereoisomers: Enantiomers
Another type of stereoisomer is a pair of molecules that are mirror images that cannot be superimposed on each other. Such stereoisomers are called enantiomers (or optical isomers), and are described as being chiral (from the Greek word cheir, χειρ, meaning “hand”; your left and right hands exhibit chirality—they are non-superimposable mirror images of each other).
Most physical, chemical, and physiological properties of two enantiomeric substances are identical. Differences between enantiomers only become evident when the substances interact with other chiral molecules or environments. (An infamous example of a physiological difference is the drug thalidomide, where one isomer causes birth defects but the other does not.)
Most chiral molecules have at least one atom that is bonded to four different groups, a chiral center. Chiral centers are often marked with an asterisk (*) in molecular structures. A carbon atom that is a chiral center is referred to as an asymmetric carbon atom or a chiral carbon atom. For example, the carbon atom in bromochloroiodomethane is a chiral center and there are two enantiomeric isomers: the two mirror-image molecules of CHBrClI are not superimposable on each other (Figures 6 and 7).
Activity 4 Analyzing Enantiomers
Study Figure 6 and Figure 7 carefully. Make certain that you understand what superimpose means and why these two mirror-image molecules cannot be superimposed.
- Based on your observations, devise a method for distinguishing one structure from the other; that is, describe how you can tell that one molecule is different from the other. Write your method in your notebook.
- To convert from (R)-bromochloroiodomethane to (S)-bromochloroiodomethane, would one or more chemical bonds need to be broken so that atoms could be re-arranged? If so, describe which bonds could be broken and which could be formed to interchange the structures.
The difference between the two enantiomers may not appear to be significant, but you would have to at least partially break and re-form two σ bonds in order to go from one enantiomer to the other. Hence, enantiomeric structures represent different substances that can be separated from one another. Separation is often difficult and requires a chiral environment: a different chiral substance that interacts differently with the left-hand molecule compared to the right-hand molecule. A macroscopic analogy is this: if all the gloves you own were mixed up, you could separate left-hand gloves from right-hand gloves by how they interact with your right hand; all gloves that fit are right-handed.
All molecules have a mirror image, but only chiral molecules have nonsuperimposable mirror images. Contrast CHBrClI with CHCl2I (dichloroiodomethane), which is shown in Figure 8. CHCl2I and its mirror image are superimposable. Thus CHCl2I is not a chiral molecule (it is said to be achiral). Two groups (the Cl atoms) bonded to the carbon atom in CHCl2I are the same, so there is no chiral center.
Generally, the easiest way to spot a chiral center is to look for four different groups bonded to an atom. The “groups” are considered in their entirety, not just the atom that is directly bonded to the chiral center. For example, a methyl group is a different group from an ethyl group, which is different from a propyl group and so forth. Hence, alkanes can be chiral, for example:
Just because you see dashed and solid wedges in a structure, do not automatically assume that you are looking at a chiral center. For example, lactic acid has an asymmetric (chiral) carbon atom (denoted by *) and is a chiral molecule, while isobutyric acid is not a chiral molecule.
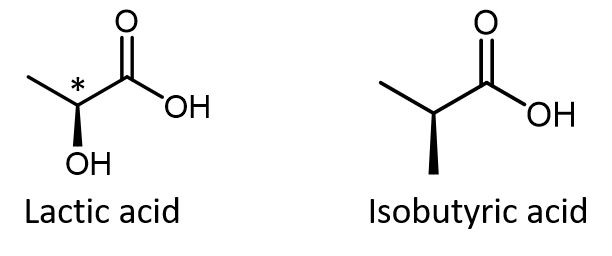
Make sure that you can recognize from the wedge-dash Lewis structure that the chiral carbon atom in lactic acid has four different groups around it but the corresponding carbon atom in isobutyric acid does not. Use these three links to access 3D rotatable models of the two enantiomers of lactic acid and the single structure of isobutyric acid. Lactic acid link 1. Lactic acid link 2. Isobutyric acid.
D11.7 Intermolecular Forces
Whether a covalent molecular substance is a solid, liquid, or gas at room temperature (or any other temperature) depends on the strengths of the attractive forces between the molecules that make up the substance. Any attractive forces between molecules are referred to as intermolecular forces (IMFs). Strengths of intermolecular forces vary widely, but IMFs are usually weaker than covalent bonds. One form of IMF, London dispersion forces, has already been discussed in Unit 1, where we indicated that boiling points reflect the energy needed to overcome intermolecular forces as molecules go from being in close proximity to each other in the liquid phase to being far apart in the gas phase. Thus, boiling points are good indicators of the relative strengths of IMFs of different molecular substances.
The boiling points of linear alkanes (CnH2n+2), with n = 1-20 are shown in Figure 9. The boiling points gradually increase with increasing length of the molecule. You should be able to explain this trend in boiling points based on LDFs.
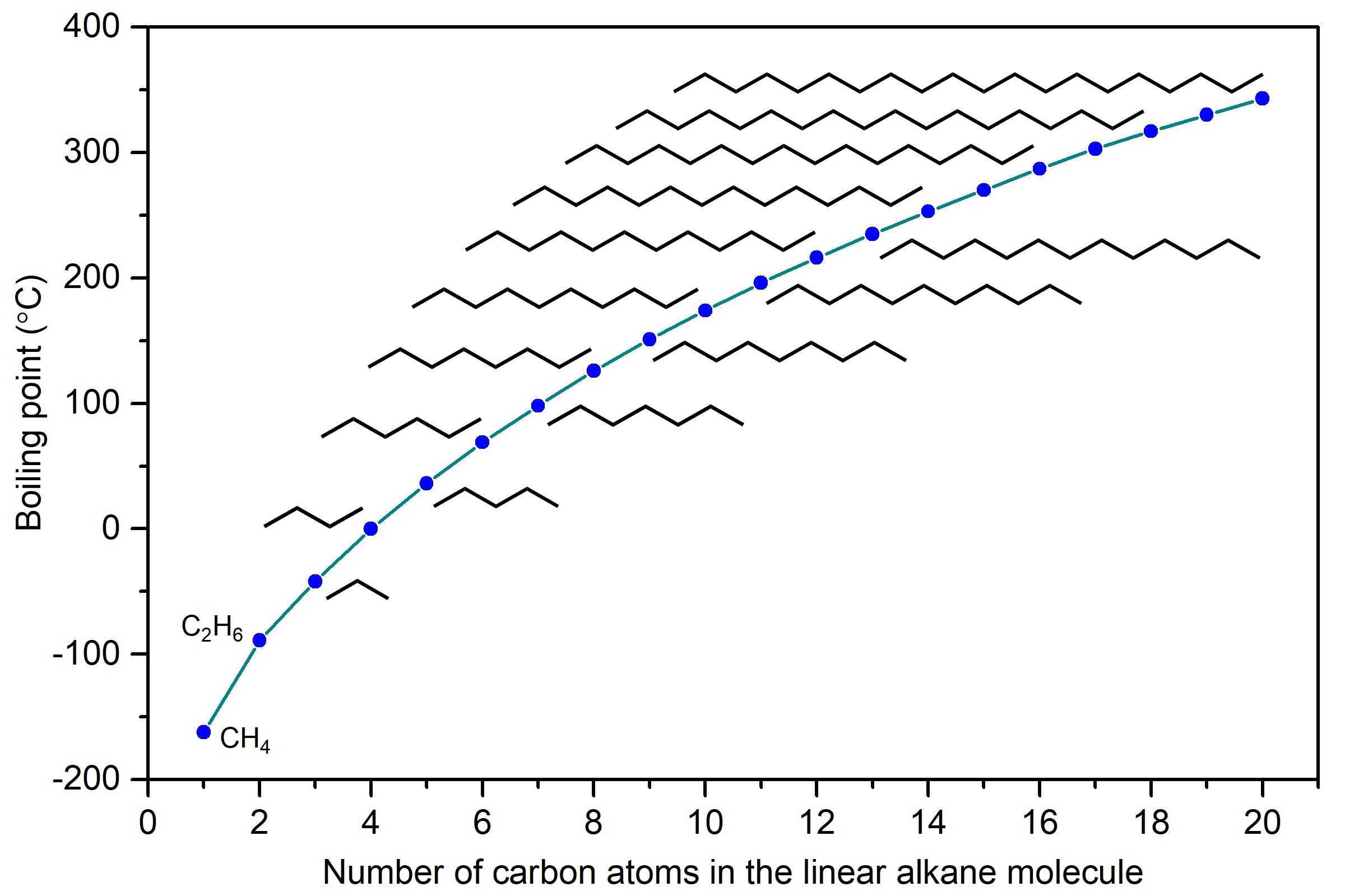
LDFs increase with number of electrons and also with ease of distortion of electron probability distribution in an atom or molecule. Because longer linear alkanes have more electrons, the LDFs increase and boiling points also increase.
Exercise 6: Match Boiling Points
Another factor that affects the strength of LDFs is molecular geometry. For example, the boiling points for n-pentane, isopentane (2-methylbutane), and neopentane (2,2-dimethylpropane) are 36 °C, 27 °C, and 9.5 °C, respectively, indicating that LDFs are greatest for n-pentane and least for neopentane.
These three molecules are constitutional isomers and therefore have same number of electrons. In n-pentane, the open linear shape provides a greater surface area between molecules when they come in contact, resulting in stronger LDFs between the molecules. Neopentane has the most compact shape of the three, yielding the smallest surface area for intermolecular contact and, hence, the weakest LDFs.
Exercise 7: Predicting Boiling Points
Another physical property that is influenced by IMFs is the viscosity of a liquid, which is a measure of the liquid’s resistance to flow. The stronger the IMFs, the more difficult it is for molecules to move past each other and the greater is the viscosity of the liquid. Alkane molecules can get quite large and Figure 9 shows that the strength of London dispersion forces is quite significant in larger alkanes (the boiling point of pentadecane C15H32 is 270°C). Hence, alkanes consisting of larger molecules also have higher viscosity. Some examples of uses for these long-chain alkanes are lubricating oil and paraffin wax. Alkanes with a chain length of 35 or more carbon atoms are found in asphalt (or bitumen), which is a sticky and highly viscous substance.
Day 11 Pre-class Podia Problem: Conformers and Isomers
Here is a structure of 2,5-dimethylhept-3-ene.
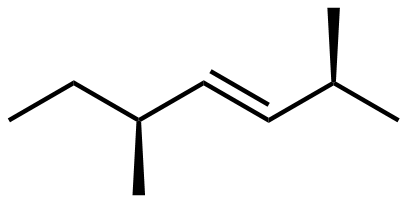
- There is one chiral carbon center in this molecule. Draw the structure and identify the chiral carbon with an asterisk (*).
- Draw a different conformation of the structure above.
- Draw the enantiomer of this molecule.
- Draw the geometric isomer of this molecule.
- Draw a constitutional isomer of this molecule that would be likely to have a higher boiling point.
- Explain why you think this constitutional isomer would have a higher boiling point.
- Draw a different conformation of this constitutional isomer.
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

