Unit Five
Day 40: Thermodynamic Properties of Voltaic and Electrolytic Cells
D40.1 Relationships among ΔrG°, K°, and E°cell
A redox reaction can be described in terms of ΔrG° and K°, and these thermodynamic variables can be related to E°cell because they all describe whether the redox reaction is reactant-favored or product-favored at equilibrium.
In a voltaic cell, the difference in Gibbs free energy between products and reactants allows the cell to do electrical work. We represent electrical work done by the cell as welec.
The charge of 1 mol electrons is known as the Faraday’s constant (F):
Hence, the total quantity of charge transferred per mole of a redox reaction is:
In this equation, n is the number of electrons transferred in the balanced redox reaction, which can be obtained from the balanced half-reactions that are added to produce the overall redox reaction. For example, in the reaction:
6 e− are transferred because each half-reaction, after multiplying by a factor to balance electrons, involves 6 e−:
| Oxidation: | 3 × (Zn(s) | ⟶ | Zn2+(aq) + 2e−) |
| Reduction: | 2 × (Au3+(aq) + 3e− | ⟶ | Au(s)) |
| Overall: | 2 Au3+(aq) + 3 Zn(s) | ⟶ | 3 Zn2+(aq) + 2 Au(s) |
When this redox reaction happens once, 6 e− are transferred. When a mole of this reaction takes place, 6 moles of e− are transferred; that is, 6 mol × 96485 C/mol = 578910 C are transferred. Hence, we have:
| welec | = | charge transferred × potential difference |
| welec | = | (nF) × (Ecell) |
If we operate a voltaic cell such that the maximum possible electrical work, welec, is done and the only work done is electrical work, then:
The negative sign makes sense because a positive Ecell indicates a spontaneous redox reaction while for ΔrG, a negative value indicates a spontaneous reaction.
If all the reactants and products are in their standard states, then the equation becomes:
This equation also links standard cell potentials to equilibrium constants, since:
Therefore:
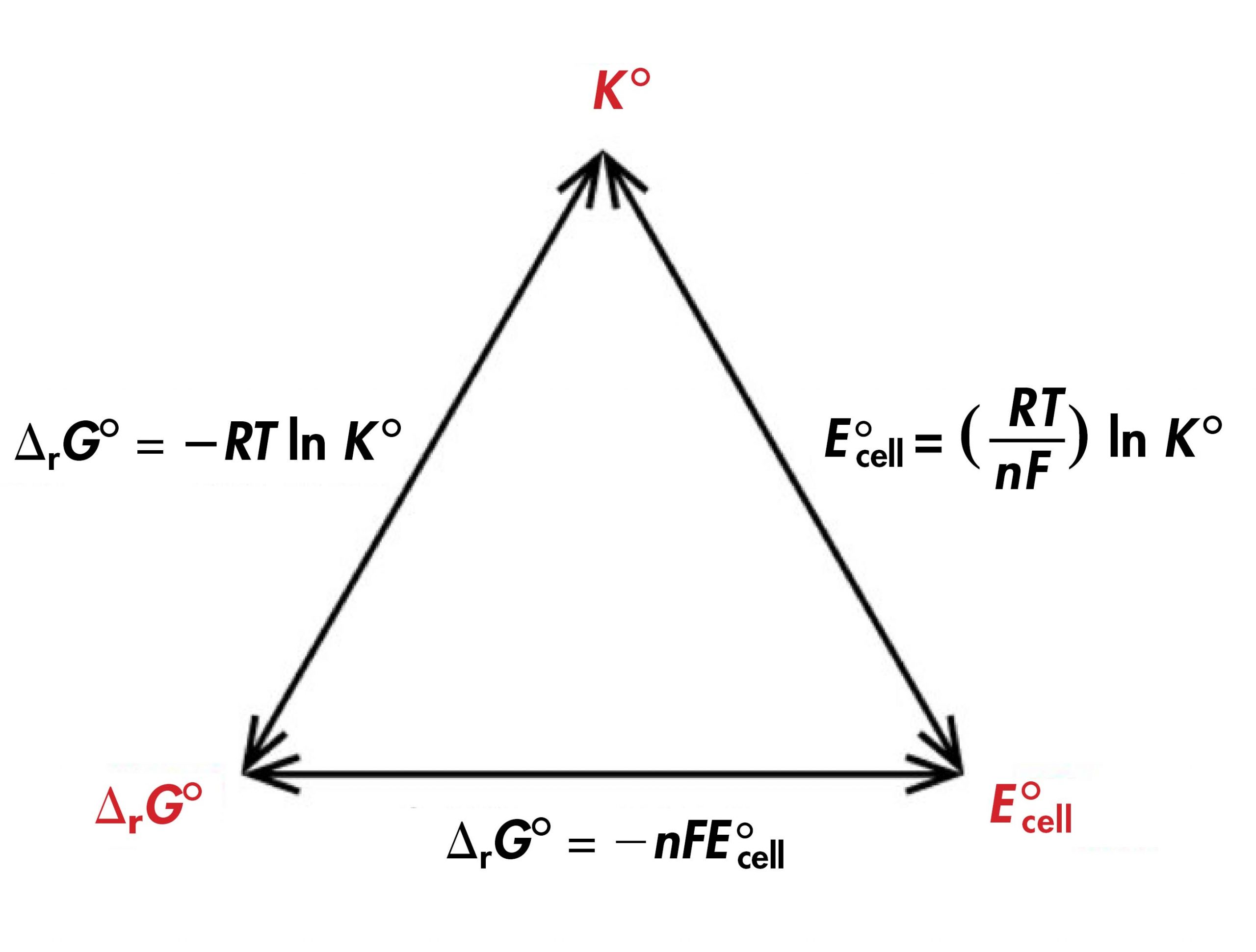
Thus, if any one of ΔrG°, K°, or E°cell is known or can be calculated for a redox reaction, the other two quantities can be determined using the relationships shown in Figure 1. Moreover, any of the three quantities can be used to determine whether a reaction is product-favored at equilibrium.
Exercise 1: Standard Gibbs Free Energy Change for a Redox Reaction
Exercise 2: Gibbs Free Energy Change and Equilibrium Constant for a Redox Reaction
D40.2 Nernst Equation
Now that the connection has been made between standard Gibbs free energy change and standard cell potentials, we can consider the situation under nonstandard conditions. Recall that ΔrG is related to ΔrG° by the reaction quotient, Q, and a similar relationship can be applied to cell potentials:
| ΔrG | = | ΔrG° + RT lnQ |
| –nFEcell | = | –nFE°cell + RTlnQ |
Rearranging the variables gives the Nernst equation:
The Nernst equation can be used to calculate the cell potential at concentrations or partial pressures that differ from standard conditions, which is often the case for voltaic cells.
Activity 1: Is a Redox Reaction Spontaneous?
D40.3 Concentration Cells
A concentration cell is a special type of voltaic cell where the electrodes are the same material but the half-cells have different concentrations of soluble species. Because one or both half-cells are not under standard-state conditions, the half-cell potentials are unequal, and there is a potential difference between the half-cells. That potential difference can be calculated using the Nernst equation.
For example, consider this concentration cell at 25°C:
The standard cell potential is 0 V because the anode and cathode involve the same reaction; however, the process is spontaneous because if equal volumes of the two half-cell solutions were mixed, the concentration of Zn2+ would change to the average of the initial concentrations, namely, to 0.30 M. The cell can do work because the concentrations of Zn2+ change.
| Oxidation: | Zn(s) | ⟶ | Zn2+(aq, 0.10 M) + 2e‾ | E°left half-cell = -0.763 V |
| Reduction : | Zn2+(aq, 0.50 M) + 2e‾ | ⟶ | Zn(s) | E°right half-cell = -0.763 V |
| Overall: | Zn2+(aq, 0.50 M) | ⟶ | Zn2+(aq, 0.10 M) | E°cell = 0 V |
The Nernst equation verifies that the process is spontaneous at the given conditions, because it shows that Ecell > 0 V:
In a concentration cell, the standard cell potential (E°cell) is always zero. In order to have a spontaneous forward reaction, and hence have a positive cell potential (Ecell), the reaction quotient Q must be less than 1 (when Q < 1, ln(Q) < 0). As the reaction proceeds, the concentrations change, Q approaches 1 and Ecell approaches 0 V.
Podia Question
The gravity cell, a variant of the Daniell cell, was described in Exercise 3 in Day 39. The cell involves oxidation of Zn(s) by Cu2+(aq). Suppose a gravity cell is constructed with 1.00 L saturated copper(II) sulfate in the bottom and 1.00 L 0.100-M zinc sulfate in the top. (There is no solid copper(II) sulfate in the cell. Assume a temperature of 25 °C.)
Write half-reactions for the overall reaction that powers the gravity cell and label them as oxidation and reduction.
Write the balanced overall cell reaction.
Calculate the standard cell potential.
Calculate the cell potential under the initial (nonstandard) conditions. The solubility of CuSO4•5H2O in water is 32 g/100 mL.
Calculate the cell potential after 99% of the copper(II) ions in the bottom cell solution has reacted. (Assume there is an excess of Zn(s).)
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

