Unit Five
Day 36: Buffer Solutions
D36.1 Buffer Solutions
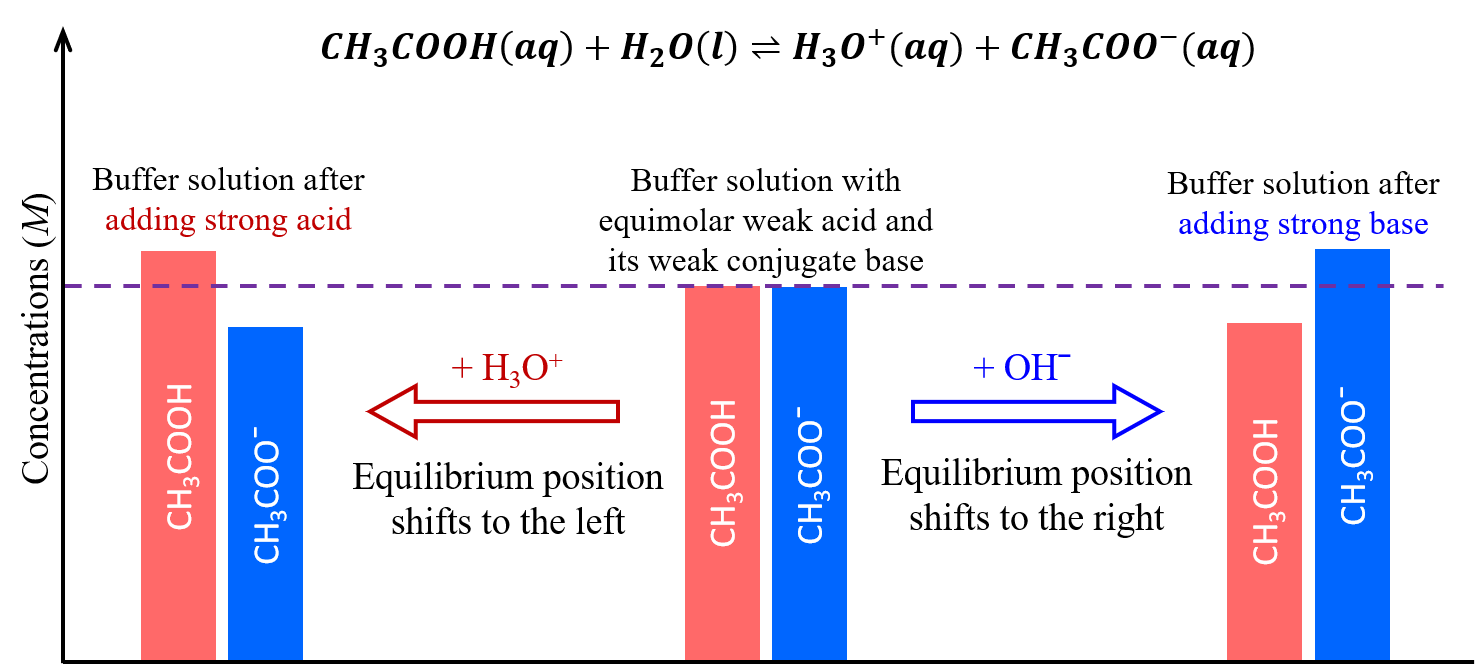
A mixture of a weak acid and its conjugate base, such as acetic acid and sodium acetate (CH3COOH + CH3COONa), or a mixture of a weak base and its conjugate acid, such as ammonia and ammonium chloride (NH3 + NH4Cl), is a buffer solution. A buffer solution resists changes in pH when small amounts of a strong acid or a strong base are added (Figure 1).

A solution of equal concentrations of CH3COOH and CH3COONa is slightly acidic because the Ka,acetic acid > Kb, acetate anion. When a strong base, such as NaOH, is added to this solution, the OH– anions react with the few H3O+ cations, decreasing concentrations of H3O+. This shifts the following equilibrium to the right:
restoring H3O+ concentration to almost the value it had before the NaOH was added. The net effect of most of the added NaOH is to convert some of the weak acid, CH3COOH, to a weak base, CH3COO−:
Hence, there is only a minimal decrease in H3O+ concentration.
When a strong acid, such as HCl, is added, the net effect of most of the added H3O+ is to convert acetate anions to acetic acid molecules:
And again, there is only a minimal increase in H3O+ concentration.
As illustrated in Figure 2, a buffer solution can moderate changes to pH because it consists of a weak acid that can react with added strong base as well as a weak base that can react with added strong acid.
The weak base and weak acid in a buffer solution are typically a conjugate acid-base pair, which maintains one dynamic equilibrium that responds to additions of other acids and bases. If they are not a conjugate acid-base pair, then there would be two dynamic equilibria at play, which significantly complicates the buffering actions.
Activity 1: pH of a Buffer Solution
Exercise 1: Characteristics of Buffer Solutions
D36.2 Henderson-Hasselbalch Equation
The ionization constant expression for a weak acid HA is:
Rearranging gives:
Taking the negative logarithm of both sides, we have:
It is much more convenient to deal with the initial concentrations of the weak acid and weak base when preparing a buffer solution. (The initial concentration is the amount of weak acid and weak base added to the solution mixture divide by the volume.) Therefore:
“x” is the increase in concentration of H3O+ as the solution reaches equilibrium (see activity 1 above) and [HA]0 and [A−]0 are the concentration of HA and A− before any reaction occurs. When the approximation that x is at least 100 times smaller than the concentrations of HA and A− is valid, we have the Henderson-Hasselbalch equation:
[latex]\text{pH} = \text{p}K_a\;+\;\text{log}\dfrac{[\text{A}^{-}]_0}{[\text{HA}]_0}[/latex]
Note that when [A−]0 = [HA]0, pH = pKa + log(1) = pKa.
The Henderson-Hasselbalch equation can be used to calculate the buffer solution pH, given the Ka and the initial concentrations, or it can be used to determine the ratio of initial concentrations of weak acid and base required to achieve a desired pH.
The Henderson-Hasselbalch equation applies only to buffer solutions in which the ratio [latex]\dfrac{[\text{A}^{-}]_0}{[\text{HA}]_0}[/latex] is between 0.1 and 10. If enough strong acid or strong base is added to the buffer solution to exceed this range, the pH begins to change significantly (in other words, the solution is no longer a buffer solution).
Exercise 2: Using the Henderson-Hasselbalch Equation to Calculate pH
D36.3 Selection of a Suitable Buffer
A buffer solution moderates changes in pH because it contains both a weak acid that can react with added strong base and a weak base that can react with added strong acid. This leads to several criteria for selecting a suitable buffer solution for a given purpose.
- The pKa of the weak acid in the buffer should be close to the desired pH of the buffer solution. According to the Henderson-Hasselbalch equation, if the concentrations of weak acid and weak base are equal, the pH of the buffer solution equals the pKa of the weak acid involved.
- A buffer solution should have approximately equal concentrations of the weak acid and weak base. A [latex]\dfrac{[\text{A}^{-}]_0}{[\text{HA}]_0}[/latex] ratio of >10 or <0.1 makes for a poor buffer solution. Figure 3 shows how the pH of an acetic acid-acetate ion buffer increases as strong base is added. The initial pH is pKa = 4.74. When pH reaches 5.74, a change of 1 pH unit, the ratio [latex]\dfrac{[\text{acetic acid}]}{[\text{acetate anion}]} = 0.11 = 11\text{%}[/latex]. After that the pH increases more rapidly and the solution no longer provides significant buffering.
![A graph is shown with a horizontal axis labeled “Added m L of 0.10 M N a O H” which has markings and vertical gridlines every 10 units from 0 to 110. The vertical axis is labeled “p H” and is marked every 1 unit beginning at 0 extending to 11. A break is shown in the vertical axis between 0 and 4. A red curve is drawn on the graph which increases gradually from the point (0, 4.8) up to about (100, 7) after which the graph has a vertical section up to about (100, 11). The curve is labeled [ C H subscript 3 C O subscript 2 H ] is 11 percent of [ C H subscript 3 CO subscript 2 superscript negative].](https://wisc.pb.unizin.org/app/uploads/sites/461/2019/11/CNX_Chem_14_06_buffer.jpg)
Figure 3. The graph, an illustration of buffering action, shows change of pH as an increasing amount of a 0.10-M NaOH solution is added to 100 mL of a buffer solution in which, initially, [CH3COOH] = [CH3COO−] = 0.10 M. - The larger the amounts (mol) of weak acid and weak base are the greater is the amount (mol) of strong base or strong acid that can be added before there is a significant change in pH .
When designing a buffer system, look for weak conjugate acid-base pairs that have pKa of the weak acid near the desired pH. Then adjust the ratio of the weak base to weak acid concentrations to achieve the exact pH desired. Make certain that the concentrations of weak base and weak acid are large enough to react with the quantities of acid or base that might be added to the buffer solution.
Activity 2: Preparing a Buffer Solution with a Desired pH
D36.4 Buffer Capacity
We can see how a buffer solution works by comparing quantitatively the pH of a buffered solution with the pH of a unbuffered solution upon addition of a strong acid or base.
Activity 3: Calculating pH Change for a Buffer Solution
Activity 4: pH Change in an Unbuffered Solution
We can see from the above activities that the change in pH is much more significant in the unbuffered solution compared to the buffered solution.
However, buffer solutions do not have an unlimited capacity to keep the pH relatively constant (Figure 4). For example, if we add sufficient strong base to a buffer that all the weak acid has reacted, no more buffering action toward the base is possible. Similarly, if we add an excess of strong acid, the weak base would all be reacted, and no more buffering action toward any additional acid would be possible. In fact, we do not even need to react away all the weak acid or base in a buffer to make significant change in pH: buffering action diminishes rapidly as a given component nears depletion. This was seen in Figure 3 in the above section, where reducing the concentration of weak acid to 11% of the concentration of weak base caused a change of 1 pH unit. The curve in Figure 3 goes up rapidly after that, indicating that the buffer has been “broken” and no longer resists changes in pH.

The buffer capacity is the amount (mol) of acid or base that can be added to a given volume of a buffer solution before the pH changes by ±1 from the pKa of the weak acid. (Recall that if equal concentrations of weak acid and conjugate base are in a buffer solution, pH = pKa.)
Buffer capacity depends on the amount (mol) of weak acid and its conjugate base that are in a buffer mixture. For example, a 1 L solution of 1.0 M CH3COOH and 1.0 M CH3COONa has a greater buffer capacity than a 1 L solution of 0.10 M CH3COOH and 0.10 M CH3COONa, even though both solutions have the same pH. The first solution has more buffer capacity because it contains more moles of acetic acid and acetate ion.
It takes 0.82 mol HCl to change the buffer pH from 4.74 to 3.74 in the first solution:
On the other hand, for the solution where the concentrations of weak acid and conjugate base are 0.10 M, it takes only one-tenth as much HCl, 0.082 mol HCl, to change the buffer pH from 4.74 to 3.74:
If a buffer solution does not have equal concentrations of weak acid and weak base, the buffer capacity when strong acid is added is different from the buffer capacity when strong base is added.
Exercise 3: Calculating Buffer Capacity
Podia Question
A solution is prepared by adding 50.0 mL 0.50-M acetic acid and 50.0 mL 0.30-M NaOH to a beaker and stirring. Is this solution a buffer solution? If so, calculate the pH of the buffer. If not, explain why the solution does not resist change in pH when 1.0 mL 0.5-M HCl is added.
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

