Unit Four
Day 32: Gibbs Free Energy and Work, Kinetic Metastability
D32.1 Gibbs Free Energy and Work
Recall that when we we talk about kinetics of a reaction, we are concerned with the rate of the reaction: how fast it goes from reactants to products. When we talk about the thermodynamics of a reaction, we are concerned primarily with the difference in energy between reactants and products, but not with the mechanism by which reactants change into products.
When the Gibbs free energy of the products is lower than that of the reactants, a reaction is said to be exergonic. Conversely, an endergonic reaction is one in which the products are higher in Gibbs free energy than the reactants.
When there is a decrease in Gibbs free energy as a reaction occurs, ΔrG equals the maximum useful work that can be done by the reaction system. ΔrG = −wmax. (The negative sign reflects the fact that w is defined as work done on the system.) Conversely, if a reaction has positive ΔrG, work must be done on the system to force the reaction to occur. The minimum work that must be done is given by ΔrG.
Activity 1: Exergonic and Endergonic Reactions
When considering a reaction under standard-state conditions the relevant thermodynamic quantity is ΔrG°. If a reaction is exergonic under standard-state conditions, ΔrG° < 0.
Exercise 1: Gibbs Free Energy and Work
Coupled Reactions
One way to allow a reactant-favored process to occur is to couple it with a reaction that is product-favored. For example, consider the recovery of aluminum from alumina (Al2O3) ore:
At least 1576.4 kJ of work must be done to change 1 mol Al2O3(s) into 2 mol Al(s) and 1.5 mol O2(g) (at 1 bar). In a modern aluminum manufacturing plant, this work is supplied electrically and the electricity is often provided by burning coal. Assuming coal to be mainly carbon, the combustion reaction is:
Thus the ΔrG° values indicate that, under standard-state conditions and ideal 100% efficiency, at least four moles of carbon/coal must burn to process each mole of Al2O3 ore. (In practice the aluminum smelting process is only 17% efficient, so it is necessary to burn nearly 6 times the theoretical amount of coal.) Coupled reactions occur simultaneously and there is a means of exchanging energy between them. The energy exchange occurs via the electric power grid in this specific case.
In other words, a reaction that is endergonic under standard-state conditions can be coupled to a separate exergonic reaction that drives the endergonic reaction (the thermodynamically unfavorable one) to occur. The ΔrG° values for the two coupled reactions are summed to yield the overall ΔrG°. For this example, multiply the second reaction by 4, add the reaction equations, and apply Hess’s Law, gives:
The overall reaction now has a negative ΔrG° and is product-favored.
Under non-standard-state conditions a reaction with ΔrG < 0 can drive a reaction with ΔrG > 0, provided energy can be transferred from one to the other.
D32.2 Gibbs Free Energy in Biological Systems
Biological organisms often couple the product-favored hydrolysis of ATP (adenosine triphosphate) to a reactant-favored reaction. Thus ATP hydrolysis reaction can be used to drive a necessary, but thermodynamically unfavorable, reaction.
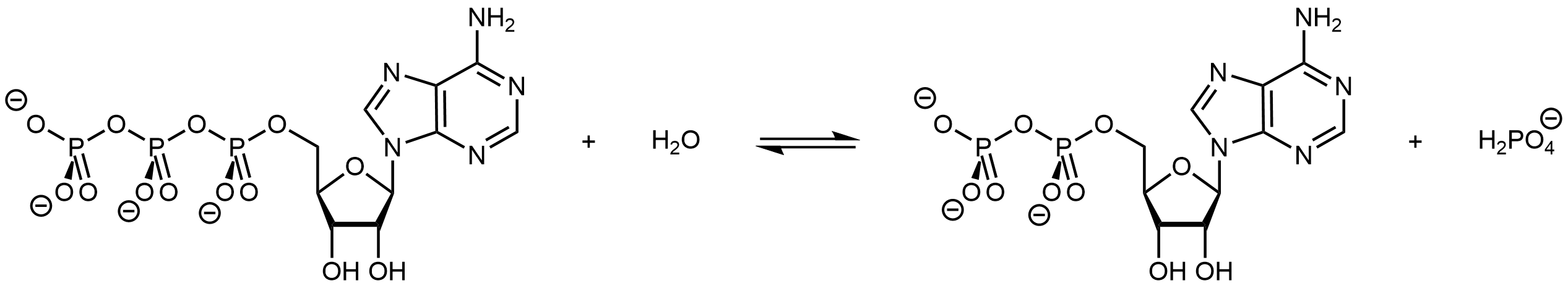
The triphosphate part of ATP is an inorganic ester. It can be formed by condensation reaction of ADP and H2PO4– with water formed as a byproduct (the reverse of the reaction shown above). ATP can be made available in an organism where an endergonic reaction needs to occur. Its hydrolysis can then be coupled with the endergonic reaction to yield a thermodynamically favorable overall reaction.
For example, ATP hydrolysis can be used to drive condensation reactions of amino acids to generate proteins as graphically illustrated by Figure 1.
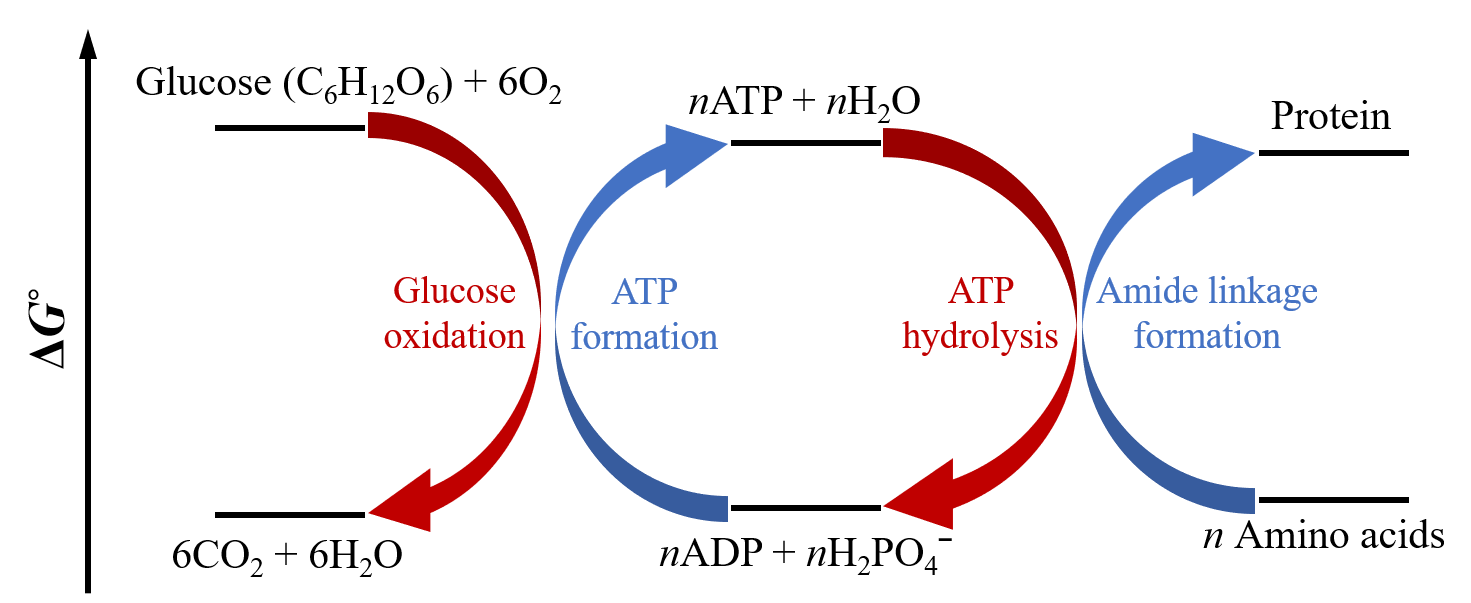
Figure 1 shows ATP formation being initially coupled to the glucose oxidization reaction:
which has close to 100x greater capability to do work than the hydrolysis of a single ATP. Hence, the equilibrium for this reaction so strongly favors the products that a single arrow is typically used in the chemical equation as it is essential irreversible. It may not be surprising that glucose and all sugars are very energetic molecules since they are the primary energy source for life.
Exercise 2: Characteristics of Exergonic Processes
D32.3 Kinetic Metastability
At a given temperature, the rate law and rate constant can be used to determine how rapidly reactants are converted to products. The equilibrium constant expression and the value of K°, on the other hand, can be used to determine the equilibrium concentrations of products relative to reactants. In other words, kinetics describes how fast equilibrium is reached, and thermodynamics describes where the equilibrium lies. However, there is not necessarily a correlation between a fast reaction and one that is product-favored at equilibrium. Both kinetics and thermodynamics are needed to characterize a chemical reaction because a useful reaction usually is one where significant quantities of products can be produced in a short time.
On the other hand, it is often true that a substance is valuable for some purpose when it is stable and does not change into some other substance. For example, iron and steel are useful for making automobiles and constructing buildings precisely because they are stable. When discussing the concept of stability, it is useful to distinguish between thermodynamic stability and kinetic metastability.
Consider a generic reaction:
Here product B has lower ΔfG° than reactant A so that ΔrG° of the forward reaction is negative. When a reaction favors products at equilibrium, we say that products are thermodynamically stable relative to reactants. In the example above, product B is thermodynamically stable compared to reactant A. However, if the activation-energy barrier (Ea) is high, at a given temperature the reaction could proceed very slowly, and reactant A would be described as being inert (unreactive). We say that compound A is kinetically metastable (or kinetically stable) relative to compound B.
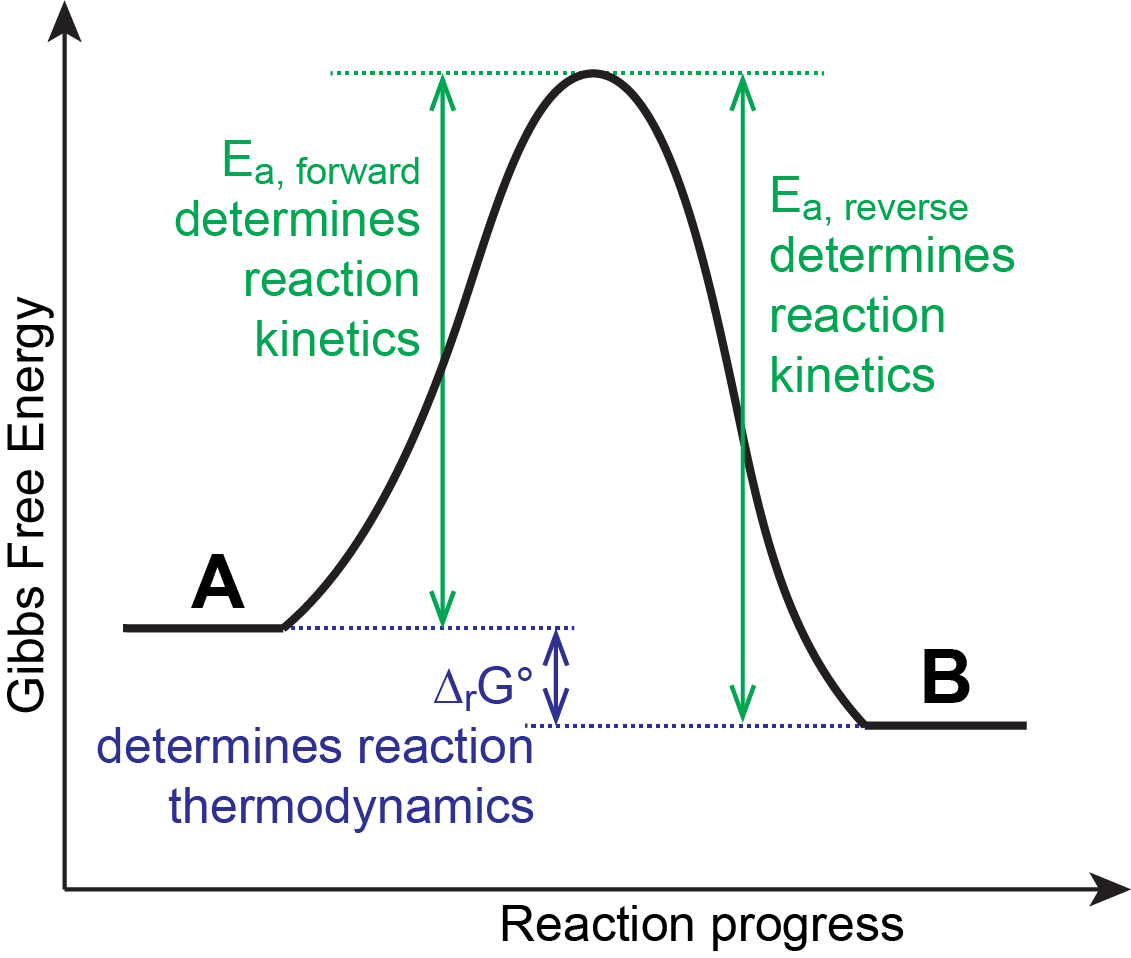
Notice that stability (and metastability) are defined by comparing one substance with another. It is possible that some other substance, say C, is even more stable than B and therefore B is thermodynamically unstable relative to C.
For example, diamond and graphite are two compounds composed solely of carbon atoms. The title of an old James Bond film, and an even older advertising slogan, says, “Diamonds Are Forever.” This implies some stability: we don’t expect the diamond in a ring to change anytime soon.
Exercise 3: Diamond and Graphite
C(s, diamond) ⟶ C(s, graphite) ΔrG° = −2.9 kJ/mol
Diamond is kinetically metastable. Thermodynamics says it should change to graphite, but the change is so slow as to be essentially undetectable at day-to-day temperatures in a human lifetime. The fact that diamond exists is due to a very large activation-energy barrier for conversion of diamond to graphite; diamond would convert to graphite at temperatures of >4500 K.
This very large Ea, as well as the facts that diamond is the hardest known solids and graphite is one of the softest, can be explained by differences in the way the atoms are bonded. In diamond, every carbon atom has sp3 hybridization and each sp3 carbon is bonded to 4 other sp3 carbon atoms at the corners of a tetrahedron. In graphite, every carbon atom has sp2 hybridization and each sp2 carbon is bonded to 3 other sp2 carbon atoms in planar sheets of connected benzene rings. Because the sheets can slide past one another relatively easily, graphite is soft and slippery.
Conversion of diamond to graphite requires breaking numerous C−C single bonds with bond energy of 356 kJ/mol. There is no easy mechanism for this conversion and so transforming diamond into graphite, or vice versa, requires almost as much energy as destroying the entire crystal lattice and rebuilding it. Therefore, once diamond is formed, it cannot convert back to graphite under normal conditions because Ea is too high: diamond is said to be metastable because its stability depends on kinetics, not thermodynamics.
Exercise 4: Kinetic Metastability
D32.4 Haber-Bosch Process
The interplay of thermodynamics and kinetics is illustrated in the industrial synthesis of ammonia. It became possible to manufacture ammonia in useful quantities in the early 20th century after the factors that influence this reaction equilibrium were understood:
Each year, ammonia is among the top 10 chemicals, by mass, manufactured in the world. It plays a vital role in the global economy. It is used in the production of fertilizers and is, itself, an important fertilizer for enhancing growth of corn, cotton, and other crops. Large quantities of ammonia are converted to nitric acid, which plays an important role in the production of fertilizers, explosives, plastics, dyes, and fibers, and is also used in the steel industry.
To be practical, an industrial process must give a large yield of product relatively quickly. One way to increase the yield of ammonia is to increase the pressure on the system, which shifts the reaction equilibrium towards the product side, increasing the concentration and partial pressure of ammonia.
At low temperatures, the rate of formation of ammonia is slow so equilibrium would be achieved more quickly at higher temperatures. However, the reaction is exothermic. Increasing the temperature to increase the rate shifts the equilibrium in the endothermic direction and lowers the product yield.
Part of the slower rate caused by operating at lower temperatures can be recovered by using a catalyst. The net effect of the catalyst on the reaction is to cause equilibrium to be reached more rapidly.
Ammonia, the reaction product, has a higher boiling point than the reactants, nitrogen and hydrogen; thus, ammonia can be condensed to a liquid at temperatures where N2 and H2 remain gases. Condensing ammonia by refrigeration of the gaseous mixture removes product, shifting the equilibrium to the right.
Exercise 5: Intermolecular Forces and Boiling Point
Ammonia has a higher boiling point than nitrogen and hydrogen because of intermolecular forces.
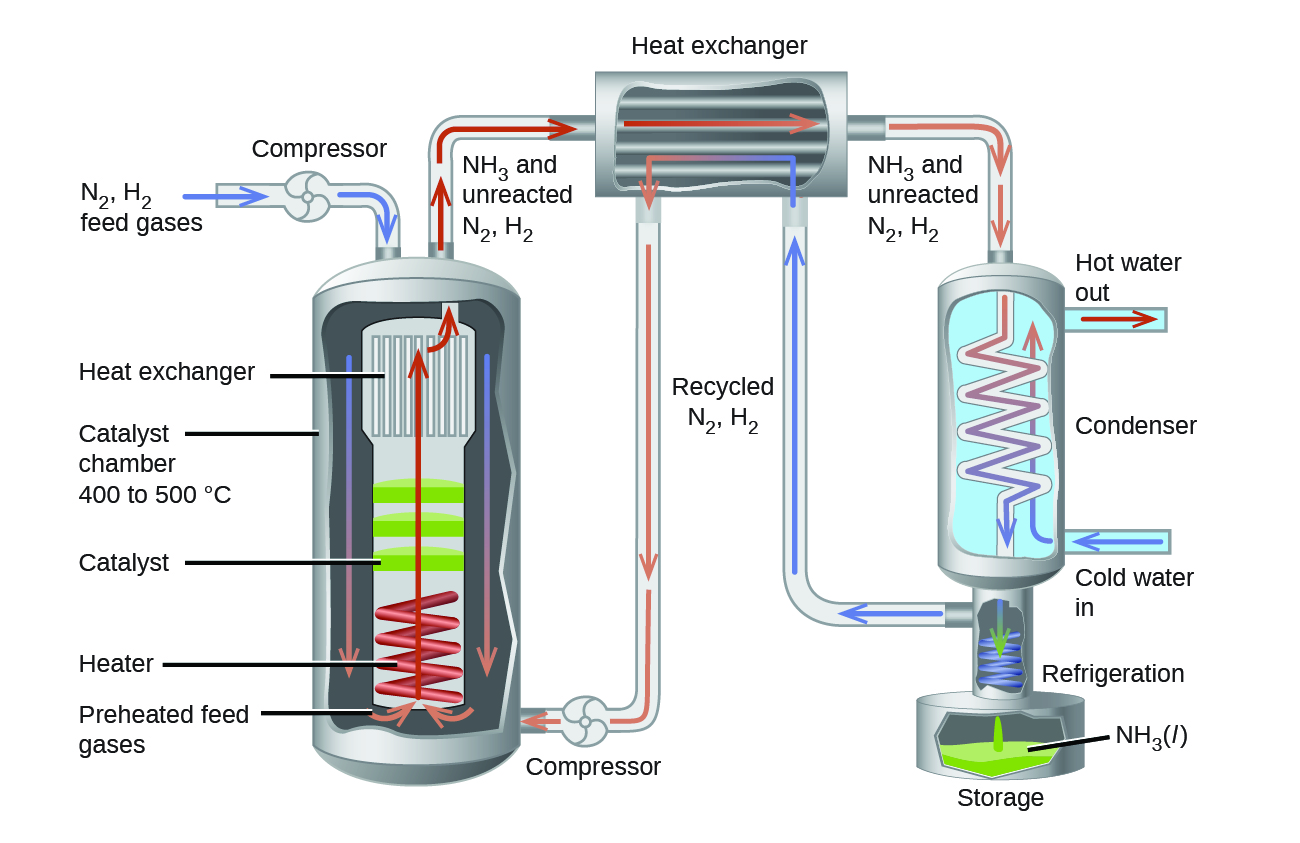
In the commercial production of ammonia, conditions are typically 400 – 500 °C and 150–250 bar. The catalyst consists of Fe3O4 mixed with KOH, Al2O3, and SiO2. This gives the best compromise among rate, product yield, and the cost of the equipment necessary to produce and contain high-pressure gases at high temperatures (Figure 3).
Podia Question
Consider the addition of HBr to 1,3-butadiene. Two products are possible: 1,2-product and 1,4-product (see left side of figure).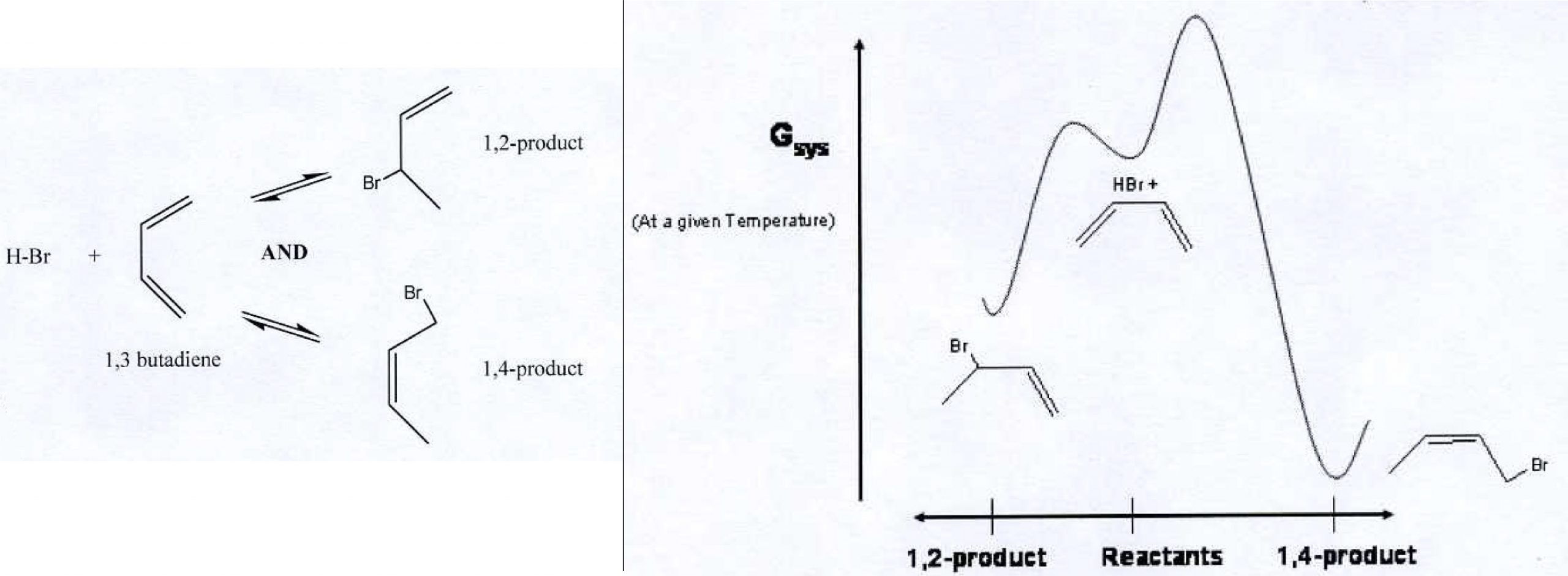
On the right side of the figure is a diagram showing Gibbs free energy as a function of reaction progress. On the horizontal axis reactants are in the middle. The 1,2-product is reached by moving left. The 1,4-product is reached by moving right.
1. Write an explanation in scientifically appropriate language for the fact that at lower temperatures the reaction produces mainly 1,2-product while at higher temperatures the reaction produces mainly 1,4-product. If any assumptions need to be made, say what they are.
2. A student says that at lower temperatures the 1,2-product is kinetically metastable compared with the 1,4-product. Is this a correct statement? Why or why not?
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

