Unit Four
Day 34: Acid-Base Reactions
D34.1 Polyprotic Acids
We can classify acids by the number of protons per molecule that they can donate in an acid-base reaction. Acids that contain one ionizable hydrogen atom per molecule are called monoprotic acids. Examples are HCl, HNO3, CH3COOH, and HCN.
Even though it contains four hydrogen atoms, acetic acid is also monoprotic because only the hydrogen atom from the carboxyl group (-COOH) reacts with bases:
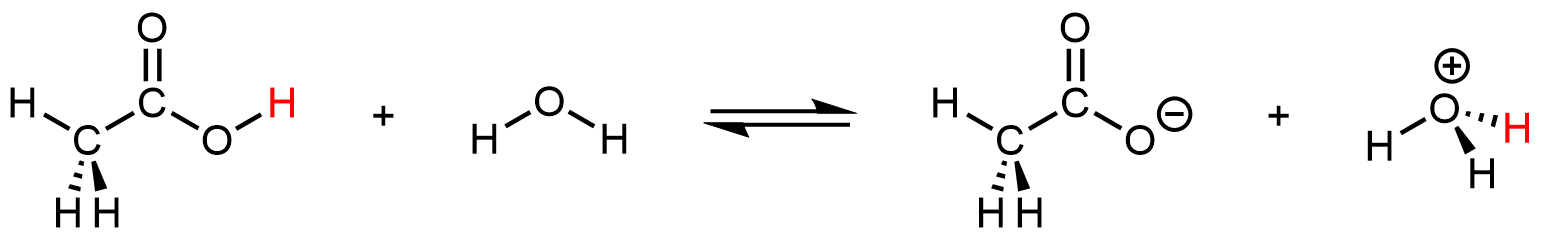
The three hydrogen atoms in the methyl group are not reactive (the C–H bonds are similar those in alkanes, which are unreactive).
In the same vein, monoprotic bases are bases that accept a single proton.
Diprotic acids contain two ionizable hydrogen atoms per molecule. The dissociation of the first H+ always takes place to a greater extent than the dissociation of the second H+. For example, sulfuric acid ionizes in two steps:
| H2SO4(aq) + H2O(l) | ⇌ | HSO4–(aq) + H3O+(aq) | Ka,1 > 102 |
| HSO4–(aq) + H2O(l) | ⇌ | SO42-(aq) + H3O+(aq) | Ka,2 = 1.1 × 10-2 |
This stepwise ionization occurs for all polyprotic acids.
A solution of a weak diprotic acid contains a mixture of acids. For example, when carbonic acid loses one H+, it yields hydronium ions and bicarbonate ions in small quantities:
The bicarbonate ion can lose an H+ to form hydronium ions and carbonate ions in even smaller quantities:
Ka(H2CO3) is larger than Ka(HCO3‾) by about four orders of magnitude (104 times larger), so H2CO3 is the dominant producer of H3O+ in the solution. This means that the concentrations of H3O+ and HCO3– are practically equal in a pure aqueous solution of H2CO3.
If Ka,1 of a weak diprotic acid at least 20 times larger than Ka,2, it is appropriate to treat the first ionization separately and calculate concentrations resulting from it before calculating concentrations of species resulting from subsequent ionization. This can simplify our work considerably because we can determine the concentration of H3O+ and the conjugate base from the first ionization, then determine the concentration of the conjugate base of the second ionization in a solution with concentrations determined by the first ionization.
Activity 1: Ionization of a Diprotic Acid
A triprotic acid is an acid that has three protons that undergo stepwise ionization: Phosphoric acid is an example:
H2PO4–(aq) + H2O(l) ⇌ HPO42-(aq) + H3O+(aq) Ka,2 = 6.3 × 10-8 M
HPO42-(aq) + H2O(l) ⇌ PO43-(aq) + H3O+(aq) Ka,3 = 4.6 × 10-13 M
Again, the differences in the ionization constants of these reactions tell us that the degree of ionization is significantly weaker in each successive step. This is a general characteristic of polyprotic acids. Here, because the successive ionization constants differ by a factor of 105-106, the calculations of equilibrium concentrations in a solution of H3PO4 can be broken down into a series of parts, similar to activity 1.
Polyprotic bases can accept more than one H+. The carbonate ion is an example of a diprotic base, because it can accept up to two protons. Solutions of alkali metal carbonates (e.g. K2CO3) are quite alkaline, due to the reactions:
HCO3–(aq) + H2O(l) ⇌ H2CO3(aq) + OH–(aq)
D34.2 Acid-Base Reactions
Mixing a solution of an acid with a solution of a base results in an acid-base neutralization reaction that produces a salt and water. The thermodynamics of an acid-base reaction dictates that the side with the weaker acid and weaker base is favored. In other words, if the weaker acid and weaker base are on the left side of an equilibrium reaction, the reaction is reactant-favored at equilibrium; if the weaker acid and weaker base are on the right side, the reaction is product-favored at equilibrium. Strengths of acids and bases are quantitatively comparable by their Ka and Kb values, which can be obtained from a reference table.
A strong acid reacts with a strong base to form a neutral solution (containing equal concentrations of H3O+ and OH‾) provided that stoichiometrically equivalent quantities of acid and base are mixed. For example:×
The salt formed, NaCl(aq), consists of Na+(aq) and Cl−(aq) , each of which has negligible acid or base strength. Hence, this equilibrium heavily favors the product side and goes essentially to completion. (Note that any soluble salt consists of aqueous ions, so the formula NaCl(aq) represents an aqueous solution consisting of the same number of Na+(aq) and Cl−(aq) ions.) If the mixture has an excess of one of the reactants, then the concentration of leftover acid (HCl) or base (NaOH) determines the pH of the solution.
A weak acid reacts with a strong base to form a salt that contains the conjugate base of the weak acid, which is usually a weak base. For example, the reaction of acetic acid with sodium hydroxide forms sodium acetate:
The equilibrium of this reaction favors the product side, and the reaction can be approximated as going to completion. Mixing stoichiometrically equivalent amounts of reactants gives a solution containing Na+(aq), which has no effect on the pH of the solution, and CH3COO–(aq), the conjugate base of acetic acid. Because the acetate anion is a weak base, the solution pH is >7 after acetic acid reacts stoichiometrically with a strong base. The weak-base reaction is:
The equilibrium constant for this reaction is the ionization constant, Kb, for the acetate anion. (Some reference tables only report ionization constants for acids; Kb can be calculated from Kw and Ka of the conjugate acid—acetic acid in this case.) Generalizing this example, when a strong base reacts stoichiometrically with a weak acid, the solution that results is basic.
Exercise 1: Relationship between Ionization Constants of an Acid and Its Conjugate Base
Exercise 2: Using a Base Ionization Constant
A strong acid reacting with a weak base forms a salt containing the conjugate acid of the weak base, which is usually a weak acid. For example, the reaction of HCl with ammonia forms ammonium chloride:
The equilibrium of this reaction favors the product side, and the reaction can be approximated as going to complection. Mixing stoichiometrically equivalent amounts of reactants gives a solution that contains Cl−(aq), which is the conjugate base of a strong acid and has no effect on the pH of the solution, and NH4+(aq), the conjugate acid of ammonia. Because the ammonium ion is a weak acid, the solution pH would be <7 after ammonia reacts stoichiometrically with a strong acid. The reaction is:
The equilibrium constant for this reaction is the ionization constant, Ka, for the acid NH4+. Generalizing this example, when a weak base reacts stoichiometrically with a strong acid, the solution that results is acidic.
Activity 2: pH of an Ammonium Salt
To predict the pH of a solution resulting from the reaction between a weak acid and a weak base, we must know both the Ka of the weak acid and the Kb of the weak base. If Ka > Kb, the solution is acidic; if Kb > Ka, the solution is basic.
Exercise 3: Is a Solution Acidic or Basic?
D34.3 Reaction Between Amphiprotic Species
Acid-base reactions can also occur between two amphiprotic species. For example, mixing a solution containing hydrogen sulfate ions (HSO4–) and a solution containing hydrogen carbonate ions (HCO3–) results in an acid-base reaction. However, if both reactants can act as either an acid or a base, which reactant is the acid and which is the base? In the example mixture, there are two possibilities:
Qualitatively, a product-favored acid-base reaction involves a stronger acid reacting with a stronger base to form a weaker acid and a weaker base. Acid and base strengths are comparable by Ka and Kb values.
In possibility I the acids are HSO4− (Ka = 1.1 × 10-2) and H2CO3 (Ka = 4.3 × 10-7) and the bases are HCO3− (Kb = 2.3 × 10-8) and SO42− (Kb = 9.1 × 10-13). The stronger acid and the stronger base are on the left side of the equation so this reaction is product-favored.
On the other hand, possibility II is reactant-favored because it produces H2SO4, a strong acid, and CO32-, a weak base with a relatively large Kb = 2.1 × 10−4 (significantly larger than the Kb for HSO4–).
Exercise 4: Acid-Base Reactions
Quantitatively, we can make use of the ionization constants to determine which reaction occurs. In possibility I,
The sum of these two equilibria gives the overall reaction for possibility I: HSO4–(aq) + HCO3–(aq) ⇌ SO42-(aq) + H2CO3(aq), and the total equilibrium constant is:
Clearly possibility I is product-favored at equilibrium because the equilibrium constant is much greater than 1.
In possibility II,
(Ka for H2SO4 is too large to measure in aqueous solution but is greater than Ka for HNO3, which is ≅20, so the value 20 is a minimum for Ka for H2SO4.) The sum of these two equilibria gives the overall reaction for possibility II: HSO4–(aq) + HCO3–(aq) ⇌ H2SO4(aq) + CO32-(aq), and the total equilibrium constant is:
Possibility II is heavily reactant-favored at equilibrium. Therefore, of the two possibilities, the reaction that proceeds (and produces products) is possibility I, where HSO4– acts as an acid and HCO3– acts as a base.
D34.4 Amino Acids
Amino acids are amphiprotic because each amino acid molecule contains a carboxylic acid group that can donate a proton and an amine group that can accept a proton. Carboxylic acids are moderately acidic, many with Ka of ~10-5. Organic amines are somewhat basic, many with Kb of ~10-4. This combination creates an interesting situation, where an acid-base reaction is possible within a single amino acid molecule:

The carboxylic acid group, with Ka = ~10-5, is a stronger acid than the protonated amine group, with Ka = Kw/Kb(amine) = 10-14/10-4 = ~10-10. The amine group (Kb = ~10-4) is a stronger base than the carboxylate anion (Kb = ~10-9). The stronger acid and stronger base are on the left side so this reaction is product-favored at pH = ~7. Hence, at the pH of a typical living organism, the amino acid is a zwitterion (German for “double ion”). A zwitterion is a species with no overall electrical charge but with separate parts that are positively and negatively charged.
The formation of a zwitterion is analogous to the acid-base reaction between methylamine (Kb = 4.4 × 10-4) and acetic acid (Ka = 1.8 × 10-5):
where the equilibrium favors products because:
Increasing the pH of an amino acid solution by adding hydroxide ions can remove the hydrogen ion from the -NH3+ group:
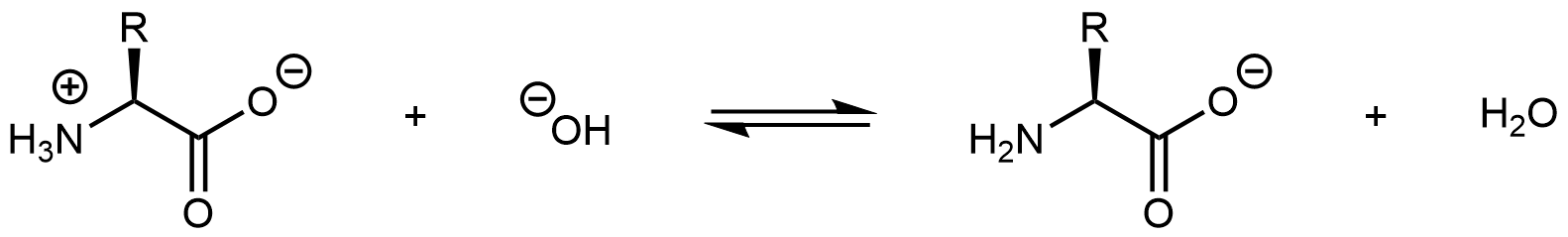
The product molecule is no longer a zwitterion. Instead, it is an anion with an overall charge of -1.
Similarly, decreasing the pH by adding strong acid to an amino acid solution protonates the -COO– part of the zwitterion:
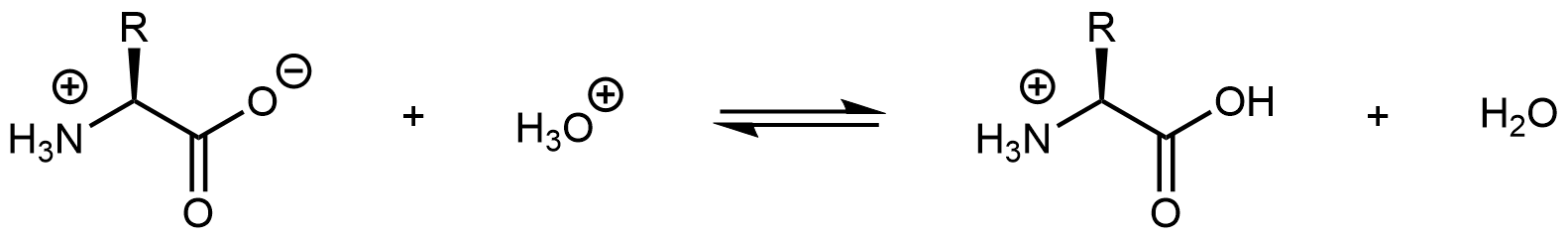
Again, the product molecule is not a zwitterion, but a cation with an overall charge of +1.
Podia Question
Write a clear, concise explanation in scientifically appropriate language for each of these correct statements.
1. When a polyprotic acid donates a hydrogen ion, the species that remains is usually a much weaker acid than was the original polyprotic acid.
2. When trichloroacetic acid reacts with hydrogen carbonate ion the equilibrium is significantly more product-favored than when acetic acid reacts with hydrogen carbonate ion.
3. When a strong base reacts with a weak acid in stoichiometrically equivalent quantity, the pH of the solution is above 7.
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

