Unit Four
Day 33: Acids and Bases
We will apply the knowledge we learned so far, e.g. thermodynamics, kinetics, molecular structure, etc., to explore two prevalent types of chemical reactions: acid-base reactions and redox/electrochemical reactions.
D33.1 Definition of Acids and Bases
According to the Brønsted-Lowry acid-base definition, a chemical species that donates a proton (hydrogen ion, H+) to another chemical species is called an acid and a chemical species that accepts a proton is a base. (Recall that when a H atom loses an electron, only the proton in the nucleus remains, so a proton is a H+ ion.) An acid-base reaction is the transfer of a proton from a proton donor (acid) to a proton acceptor (base).
The chemical species that remains after an acid has donated a proton is called the conjugate base of that acid. Consider these examples of the generic reaction acid + H2O ⇌ conjugate base + H3O+:
H2SO4 + H2O ⇌ HSO4- + H3O+
HSO4- + H2O ⇌ SO42- + H3O+
NH4+ + H2O ⇌ NH3 + H3O+
Similarly, the chemical species that forms after a base accepts a proton is called the conjugate acid of that base. Consider the following examples of the generic reaction base + H2O ⇌ conjugate acid + OH-:
S2- + H2O ⇌ HS- + OH-
CO32- + H2O ⇌ HCO3- + OH-
F- + H2O ⇌ HF + OH-
From these examples, we can see that the conjugate acid and conjugate base are paired: the conjugate base of an acid has that acid as its conjugate acid. For instance, NH3 is the conjugate base of NH4+, while NH4+ is the conjugate acid of NH3. Similarly, F‾ is the conjugate base of HF, while HF is the conjugate acid of F‾.
You may have noticed in earlier sections that we wrote H+(aq) to represent hydrogen ions in aqueous solution, while in the preceding reactions we have written H3O+. A H+ ion, a proton, is much smaller than any other cation and therefore is a highly concentrated positive charge that strongly attracts water-molecule dipoles and forms strong hydrogen bonds. Thus, when a Brønsted-Lowry acid transfers a proton to water, more than a single water molecule accepts the proton. Experiments show that as many as six water molecules may be involved, which would make the formula of H+(aq) H13O6+, but the structure changes continually as water molecules move about in the liquid. For Brønsted-Lowry acid-base reactions, we will use H3O+(aq) to emphasize that a proton has been transferred to water and to represent the more complicated actual structure. In general, H+(aq) is appropriate to represent a proton surrounded by many water molecules.
Finally, Brønsted-Lowry acid-base reactions are very fast. Their equilibrium is established quickly and this equilibrium is an important aspect when we consider acid and base strengths later.
Exercise 1: Brønsted-Lowry Acids and Bases
D33.2 Autoionization of Water
In the example reactions above, there are also two other conjugate acid-base pairs: H2O is the conjugate base of its conjugate acid H3O+, and H2O is the conjugate acid of its conjugate base OH-. (However, note that H3O+ is not the conjugate acid of OH-; these two species are not a conjugate acid-base pair because their structures do not differ by a single H+.)
Hence, H2O can react as an acid or a base depending on the other species involved in the reaction. In pure water, H2O acts as both acid and base—a very small fraction of water molecules donate protons to other water molecules:
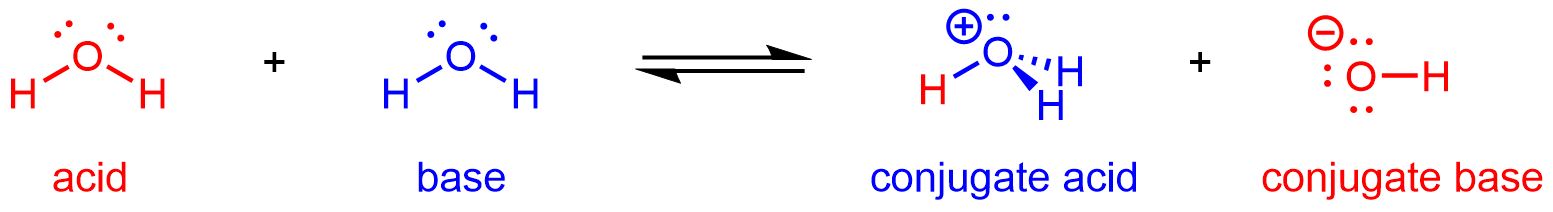
This type of reaction, in which a substance ionizes when one molecule of the substance reacts with another molecule of the same substance, is referred to as autoionization.
Pure water undergoes autoionization to a very slight extent: only about two out of every 109 molecules are ionized at 25 °C. The [H3O+]e and [OH-]e give an autoionization constant for water, Kw = 1.0 × 10−14 at 25 °C. Because it is the mathematical product of concentrations of two ions, it is also called the ion-product constant for water.
Activity 1: Autoionization of Water
Exercise 2: Autoionization of Water
Water is an example of an amphiprotic chemical species a molecule that could either gain a proton or lose a proton in an acid-base reaction. Amphiprotic species are also amphoteric, a more general term for a species that may act either as an acid or a base by any definition (not just the Brønsted-Lowry definition). For example, the bicarbonate ion is also amphoteric:
HCO3-(aq) + H2O(l) ⇌ H2CO3(aq) + OH-(aq)
Activity 2: Amphiprotic Species
D33.3 pH and pOH
The concentrations of H3O+ and OH- in a solution are important for the solution's acid-base properties and often affect the chemical behaviors of other solutes. A solution is neutral if its [H3O+]e = [OH-]e; acidic if its [H3O+]e > [OH-]e; and basic if its [H3O+]e < [OH-]e.
A common means of expressing values that span many orders of magnitude is to use a logarithmic scale. One such scale is based on the p-function:
where “X” is the quantity of interest and “log” is the base-10 logarithm. The pH of a solution is therefore defined as:
The reason for dividing by the units "mol/L" (M) is that [H3O+] has units of mol/L and taking the logarithm of a unit makes no sense. From here on we will assume that you are aware that only the numeric value of a concentration (or other quantity) needs to be used as the argument of a logarithm and we will not explicitly divide by the units.
If a pH value is known, the concentration of hydronium ions can be calculated:
Here we assume that you know that units are required for the concentration obtained from this equation and the units are mol/L. Similarly, the hydroxide ion concentration may be expressed as pOH:
Finally, the relation between pH and pOH can be derived from the Kw expression:
| Kw | = | [H3O+]e[OH-]e |
| -log(Kw) | = | -log([H3O+]e[OH-]e) |
| pKw | = | -log([H3O+]e) + (-log([OH-]e)) |
| pKw | = | pH + pOH |
At 25 °C:
Therefore, at this temperature:
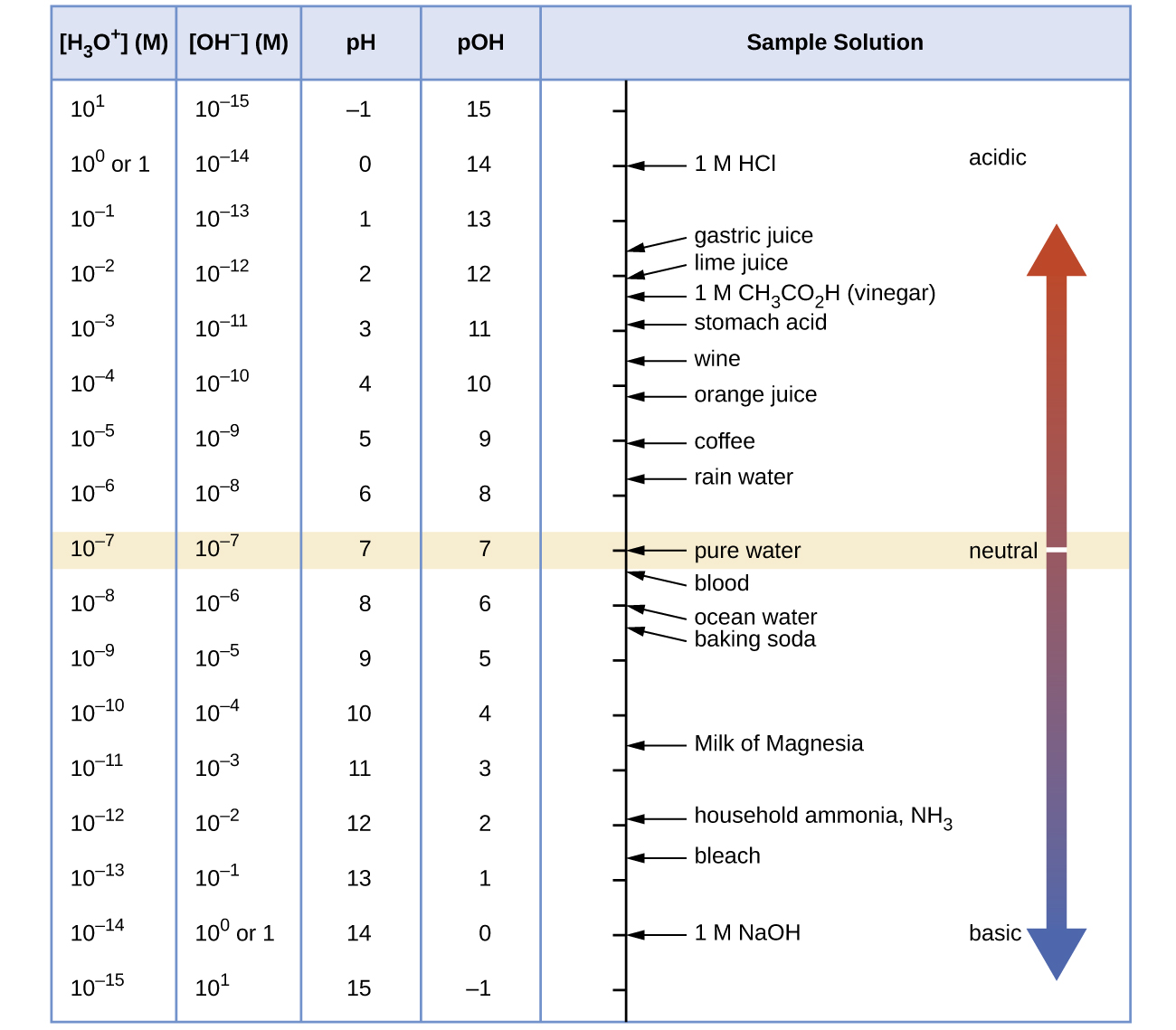
| Classification | Relative Ion Concentrations | pH at 25 °C |
|---|---|---|
| acidic | [H3O+] > [OH−] | < 7 |
| neutral | [H3O+] = [OH−] | 7 |
| basic | [H3O+] < [OH−] | > 7 |
Because Kw is temperature dependent, the correlations between pH values and the acidic/neutral/basic adjectives are different at different temperatures. For example, [H3O+] in pure water at 80 °C is 4.9 × 10−7 M, which corresponds to pH and pOH values of:
pOH = -log[OH-]e = -log(4.9 × 10−7) = 6.31
At this temperature, neutral solutions have pH = pOH = 6.31, acidic solutions have pH < 6.31 and pOH > 6.31, and basic solutions have pH > 6.31 and pOH < 6.31. This distinction can be important when studying certain processes that occur at temperatures other than 25 °C, such as acid-base reactions in the human body where temperatures are typically 37 °C.
Unless otherwise noted, references to pH values are presumed to be those at 25 °C. Figure 1 shows the relationships among [H3O+], [OH−], pH, and pOH, and gives values for these properties for some common substances.
Activity 3: pH and Relative Strengths of Acids
Exercise 3: pH of Aqueous Solutions
The acidity of a solution is typically determined by measuring its pH. The pOH of a solution is not usually measured, but it is easily calculated from an experimentally determined pH value. The pH of a solution can be directly measured using a pH meter (Figure 2), or visually estimated using colored indicators (Figure 3).


D33.4 Acid Constant Ka and Base Constant Kb
The relative strengths of acids and bases may be determined by comparing the equilibrium constants for their ionization reactions. For the reaction of a generic acid, HA, in water:
we write the acid ionization constant (Ka) expression as:
(Although water is a reactant in the reaction, it is also the solvent with its phase indicated as "l", so we do not include [H2O] in the expression.)
An acid with a larger Ka would have a larger concentration of H3O+ and A− relative to the concentration of the nonionized acid, HA. Thus a stronger acid, which ionizes to a greater extent, has a larger ionization constant than a weaker acid.
For example, these data on acid ionization constants:
| CH3COOH(aq) + H2O(l) | ⇌ | CH3COO-(aq) + H3O+(aq) | Ka = 1.8 × 10-5 |
| HNO2(aq) + H2O(l) | ⇌ | NO2-(aq) + H3O+(aq) | Ka = 7.4 × 10-4 |
| HSO4-(aq) + H2O(l) | ⇌ | SO42-(aq) + H3O+(aq) | Ka = 1.1 × 10-2 |
indicate that the order of acid strength is: acetic acid (CH3COOH) is a weaker acid than nitrous acid (HNO2) which is itself weaker than hydrogen sulfate ion (HSO4-).
We can consider the strength of a base (B) similarly by considering the extend that it will form hydroxide ions in an aqueous solution:
where the base ionization constant (Kb) expression is:
A stronger base ionizes to a greater extent than does a weaker base. Therefore, a stronger base has a larger Kb than a weaker base.
Notice that Ka and Kb provide a quantitative measure of acid and base strengths—significantly more accurate than qualitative descriptions of "strong acid" or "weak acid".
Consider the ionization reactions for a conjugate acid base pair, HA and A−:
A-(aq) + H2O(l) ⇌ HA(aq) + OH-(aq) [latex]K_{\text{b}} = \dfrac{[\text{HA}]_e[\text{OH}^{-}]_e}{[\text{A}^{-}]_e}[/latex]
Adding these two chemical equations yields the equation for the autoionization of water:
When two equilibria sum to a third equilibrium, the product of the first two equilibrium constants equals the third equilibrium constant:
For example, at 25 °C, Ka of acetic acid (CH3COOH) is 1.8 × 10−5 M, and Kb of its conjugate base, acetate anion (CH3COO-), is 5.6 × 10−10 M. The product of these two constants is indeed equal to Kw:
This relationship tells us that stronger acids form weaker conjugate bases, and weaker acids form stronger conjugate bases.
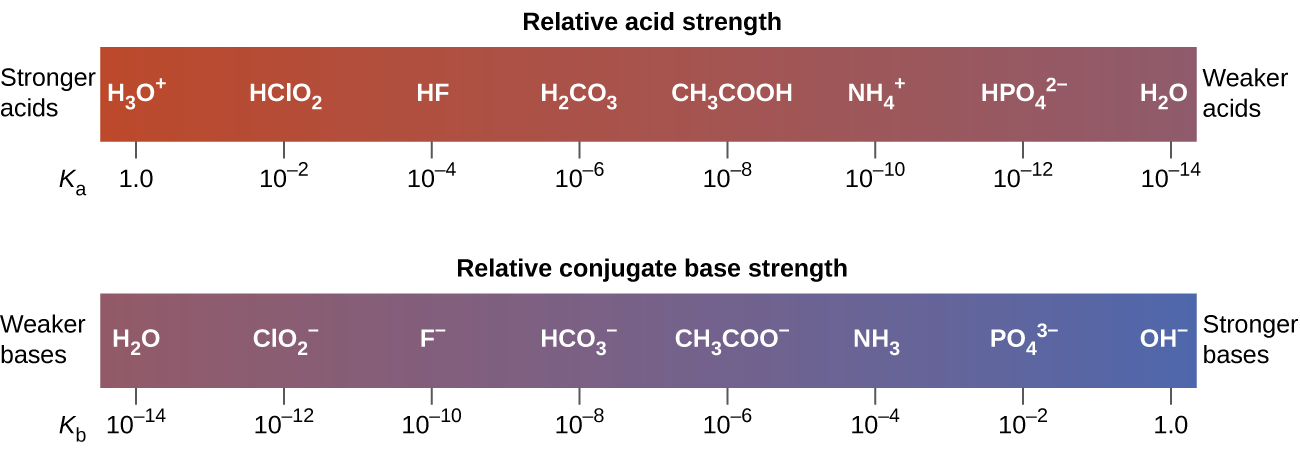
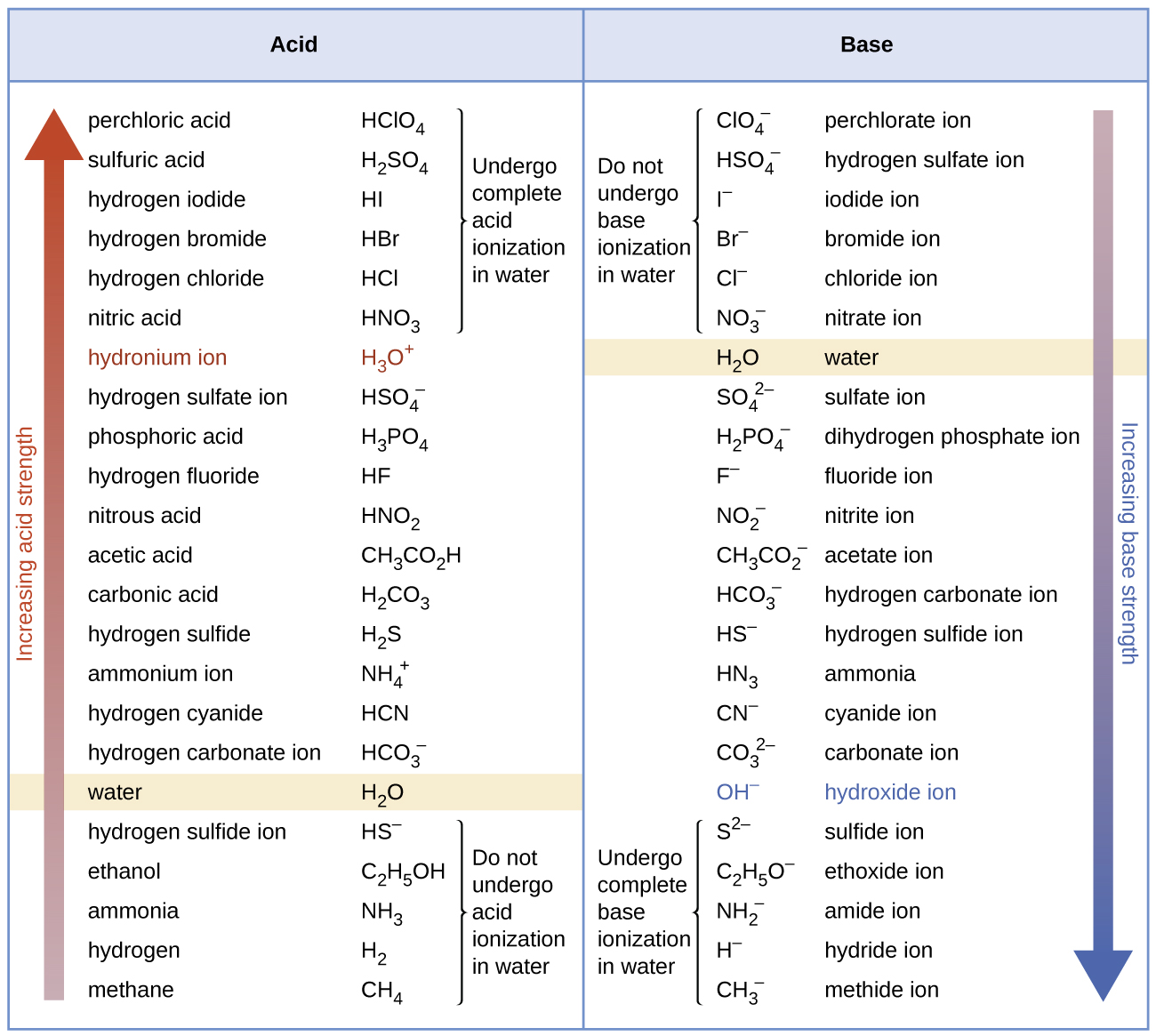
Although "strong" and "weak" are relative terms, generally, we refer to acids stronger than H3O+ as strong acids, and bases stronger than OH- as strong bases. Because strong acids and strong bases are completely ionized in aqueous solutions, the concentration of nonionized acid or base is essentially zero. For example, in a 0.10-M solution of HCl, [HCl]e = 0, [H30+]e = 0.10 M, and [Cl−]e = 0.10 M.
A consequence of this complete ionization is that in aqueous solution there is no way to tell whether one strong acid is stronger than another: HCl, HBr, and HI all are completely ionized. This is known as the leveling effect of water. However, when dissolved in some other solvents, these acids do not ionize completely. The extent of ionization increases in the order HCl < HBr < HI, and so HI is the strongest of these acids. Water exerts a similar leveling effect on strong bases.
Many acids and bases are considered "weak". A solution of a weak acid in water is a equilibrium mixture of the nonionized acid, hydronium ion, and the conjugate base of the acid.
Activity 4: Determining Ka
Activity 5: Using Ka to Calculate Concentrations
The percent ionization of a weak acid is another measure of the strength of an acid, HA:
A stronger acid, with a higher Ka, has higher percent ionization than a weaker acid (for the same concentration).
The percent ionization for a solution of a weak acid increases with decreasing acid concentration; this can be seen by applying Le Chatelier's principle to the ionization equilibrium:
HA(aq) + H2O(l) ⇌ A-(aq) + H3O+(aq)
Increasing the solution volume for a given quantity of HA added causes the equilibrium to shift to the product side to partially counteract the decrease in total solute concentration.
Exercise 4: Percent Ionization
D33.5 Acid Strength and Molecular Structure
Acid-base reactions, like many other chemical reactions, involve breaking and forming bonds. Hence, we can use our chemical understanding of molecular structure and stability to understand what makes some acids stronger than others.
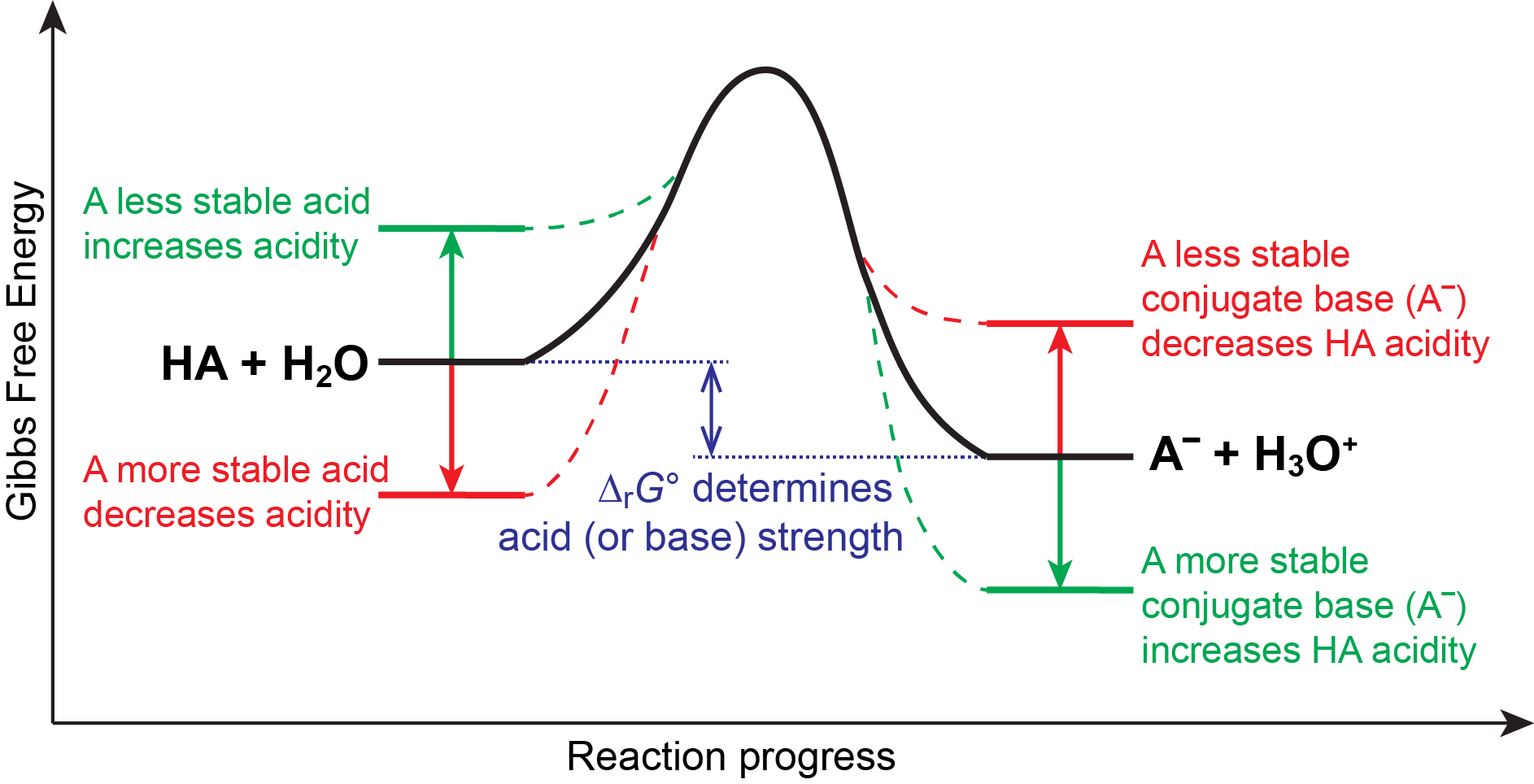
We know that an equilibrium favors the thermodynamically more stable side of the reaction, and that the magnitude of the equilibrium constant reflects the energy difference (ΔrG°) between the reactants and products. Therefore, in an acid-base equilibrium, the equilibrium always favors the side with weaker acid and base (these are the more stable species). Consequently, anything that stabilizes the conjugate base of an acid will necessarily make that acid stronger, and anything that stabilizes the acid will make it a weaker acid. This idea is illustrated in Figure 6.
Bond Strength
Let's consider a generic acid:
In general, the stronger the H–A bond is, the more stable the HA molecule is, and the less acidic the substance is. This effect is illustrated by the hydrogen halides:
| Relative Acid Strength | HF | HCl | HBr | HI |
|---|---|---|---|---|
| H–X Bond Enthalpy (kJ/mol) | 566 | 431 | 366 | 299 |
| pKa | 3.2 | −6.1 | −8.9 | −9.3 |
Note that the "bond enthalpy" values are associated with a different bond breaking reaction, which produces a hydrogen atom instead of a hydrogen ion:
Nonetheless, bond strengths correlate with acid strength. As you go down the halide group, the overlap between the hydrogen 1s orbital and the valence orbital of the halogen atom decreases, and the H-X bond enthalpy decreases, indicating weaker bonds. As a result, HX acid strength increases as you go down the group.
A similar trend is observed for other groups. For example, for group 16:
| H2A ⇌ HA- + H+ | ||||
| H2O | H2S | H2Se | H2Te | |
| pKa | 14.0 | 7.0 | 3.9 | 2.6 |
Stabilizing the Excess Charge on Conjugate Base
Electronegativity
When the conjugate base is negatively charged, factors that stabilize the excess negative charge on the conjugate base favor the dissociation of the acid and make the acid a stronger acid. For example, the relative acidity of the acids with second row elements is:
| HnA ⇌ Hn-1A- + H+ | ||||
| CH4 | NH3 | H2O | HF | |
| pKa | 50 | 36 | 14.0 | 3.2 |
Consider the compounds at both ends of this series: methane and hydrogen fluoride. The conjugate base of CH4 is CH3-, and the conjugate base of HF is F−. Because fluorine is much more electronegative than carbon, fluorine can better stabilize the extra negative charge in the F− anion than carbon can stabilize the extra negative charge in the CH3− anion. Consequently, HF can dissociate and form H+ and F− to a much greater extent than CH4 can form H+ and CH3-, making HF a much stronger acid than CH4.
The same trend is predicted by analyzing the acids as well: as the electronegativity of A in HnA increases, the A–H bond becomes more polar, favoring dissociation to form Hn-1A− and H+. Due to both the increasing stability of the conjugate base and the increasing polarization of the A–H bond in the acid, acid strengths of binary hydrides increase as we go from left to right across a row of the periodic table.
Electron Delocalization
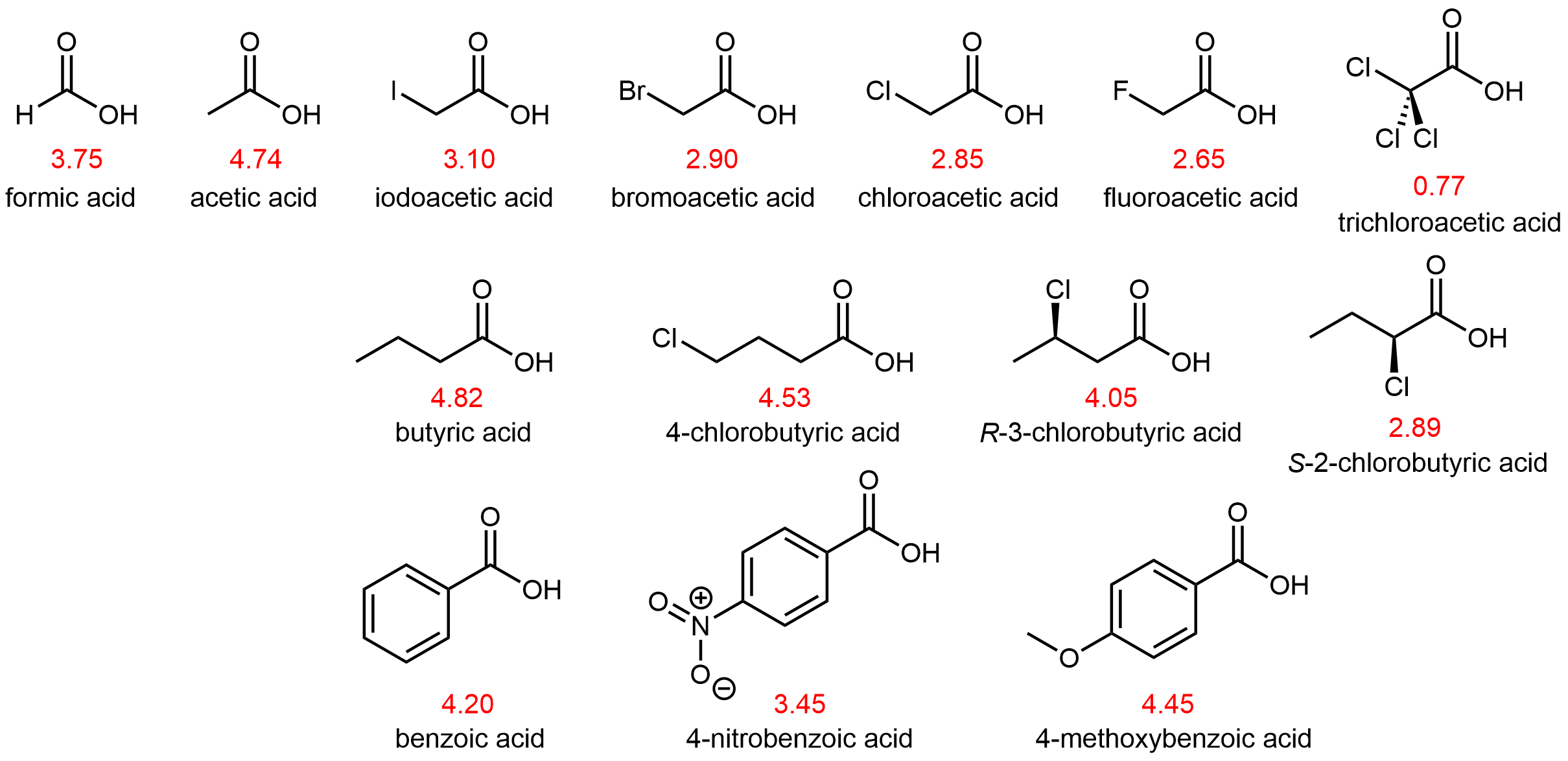
The pKa values of some carboxylic acids are shown in Figure 7.
The bond that breaks in a carboxylic acid is the O–H bond:
An O–H bond also breaks when an alcohol acts as an acid in an acid-base reaction:
However, when we compare the carboxylic acid pKa's with those of comparable alcohols, such as:
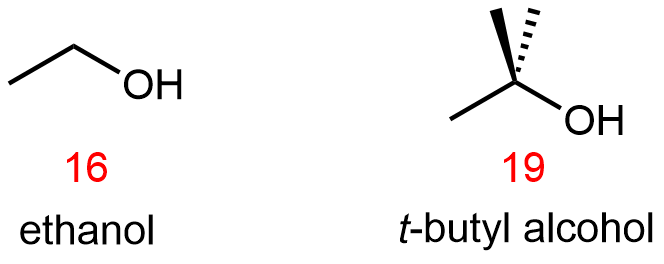
it is clear that carboxylic acids are stronger acids than alcohols by over ten orders of magnitude. Why should the presence of a carbonyl group adjacent to a hydroxyl group have such a profound effect on the acidity of the hydroxyl proton?
Both the carboxylic acid and its conjugate base, carboxylate anion, are stabilized by having additional resonance structures that delocalize electron densities. However, the stabilization in carboxylate anion is much greater because the two major resonance structures have equal contributions to the resonance hybrid, and the extra electron density (negative charge) is shared equally between the two oxygen atoms, which have high electronegativity. This stabilization leads to a markedly increased acidity of carboxylic acids.
Inductive Effect
Atoms (or groups of atoms) in a molecule that are not directly bonded to the acidic H can also influence the molecule's acidity. They can do so via an inductive effect, that is, they induce a polarization in the distribution of electrons within the molecule. This can be seen by studying the structures in Figure 7 above. Electronegative substituents (F, Cl, Br, I) near the carboxyl group act to increase the acidity of the carboxylic acid. For example, fluoroacetic acid, CH2FCOOH, is significantly more acidic than acetic acid, CH3COOH.
The magnitude of the inductive effect depends on both the nature and the number of halogen substituents, as shown by the pKa values for several acetic acid derivatives:
| CH3COOH | CH2ClCOOH | CHCl2COOH | CCl3COOH | CF3COOH | |
| pKa | 4.74 | 2.85 | 1.35 | 0.77 | 0.52 |
Fluorine, which is more electronegative than chlorine, causes a larger inductive effect as it draws electron density away from the acidic proton in the carboxyl group. Moreover, having three halogens causes a larger inductive effect than having two or one. Note that inductive effects can be quite significant. For instance, replacing the –CH3 group of acetic acid by a –CF3 group results in a almost 10,000-fold increase in acidity.
In another example, the acidity of hypohalous acids (HOX ⇌ OX‾ + H+) varies by about three orders of magnitude due to the difference in electronegativity of the halogen atoms:
| HOX | Electronegativity of X | pKa |
|---|---|---|
| HOCl | 2.73 | 7.2 |
| HOBr | 2.64 | 8.5 |
| HOI | 2.11 | 10.5 |
As the electronegativity of X increases, the electrons are drawn more strongly toward the halogen atom and, in turn, away from the H in the O–H bond, thus weakening the O–H bond, enhancing ionization of hydrogen as H+ and making the acid stronger.
Oxoacids
The acidity of oxoacids, with the general formula HOXOn (with n = 0−3), depends strongly on the number of terminal oxygen atoms attached to the central atom X (Figure 8) due to both an inductive effect and increased stabilization of the conjugate base.
Because oxygen is the second most electronegative element, adding terminal oxygen atoms (oxygen atoms only bonded to the central atom) causes electron densities to be drawn away from the O–H bond, thereby increasing the strength of the acid. Figure 9 shows how the H atom becomes steadily more blue from HClO to HClO4, making it easier for the acid to lose the hydrogen as an H+ ion.

Also important is the effect of electron delocalization that stabilizes the extra negative charge in the conjugate base. For example, in the chlorite anion (ClO2‾), the excess electron density is delocalized equally over both oxygen atoms, whereas in the hypochlorite ion (ClO−), the negative charge is largely localized on a single oxygen atom:
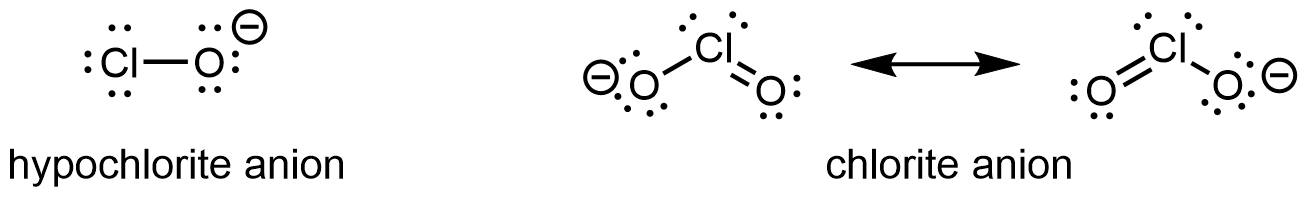
As a result of this stabilization of the conjugate base, as well as additional inductive effect, chlorous acid is more than 200,000 times stronger than hypochlorous acid.
The inductive effect is additionally responsible for the trend in the acidities of oxoacids that have the same number of oxygen atoms as we go across a row of the periodic table. For example, H3PO4 is a weak acid, H2SO4 is a strong acid, and HClO4 is one of the strongest acids known. The number of terminal oxygen atoms increases steadily across the row, consistent with the observed increase in acidity. In addition, the electronegativity of the central atom increases steadily from P to S to Cl, which causes more electron densities to be drawn from OH group to the central atom, weakening the O–H bond and increasing the strength of the oxoacid.
Podia Question
Arrange these acids in order of decreasing acid strength (smallest pKa first, largest pKa last). Analyze the factors that affect the strength of each acid and based on that analysis explain the order of acidity.
CH3CH2OH CF3COOH HOClO3 CH2BrCOOH HOClO2
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

