Module 7: Blood and Blood Vessels
Learning Objectives:
By the end of this module, students will be able to:
- Describe the components of blood.
- Compare and contrast the formed elements of blood and their functions.
- Explain blood types, blood donor compatibility, and the Rh factor.
- Describe the three layers that make up blood vessels.
- Compare and contrast the structure of the walls of the different types of arteries, veins, and capillaries.
- Explain how blood returns to the heart despite low blood pressure in veins.
- Explain the importance of collateral circulation.
Terms to Know
|
Blood
|
Vessels
|
Fluid Connective Tissue
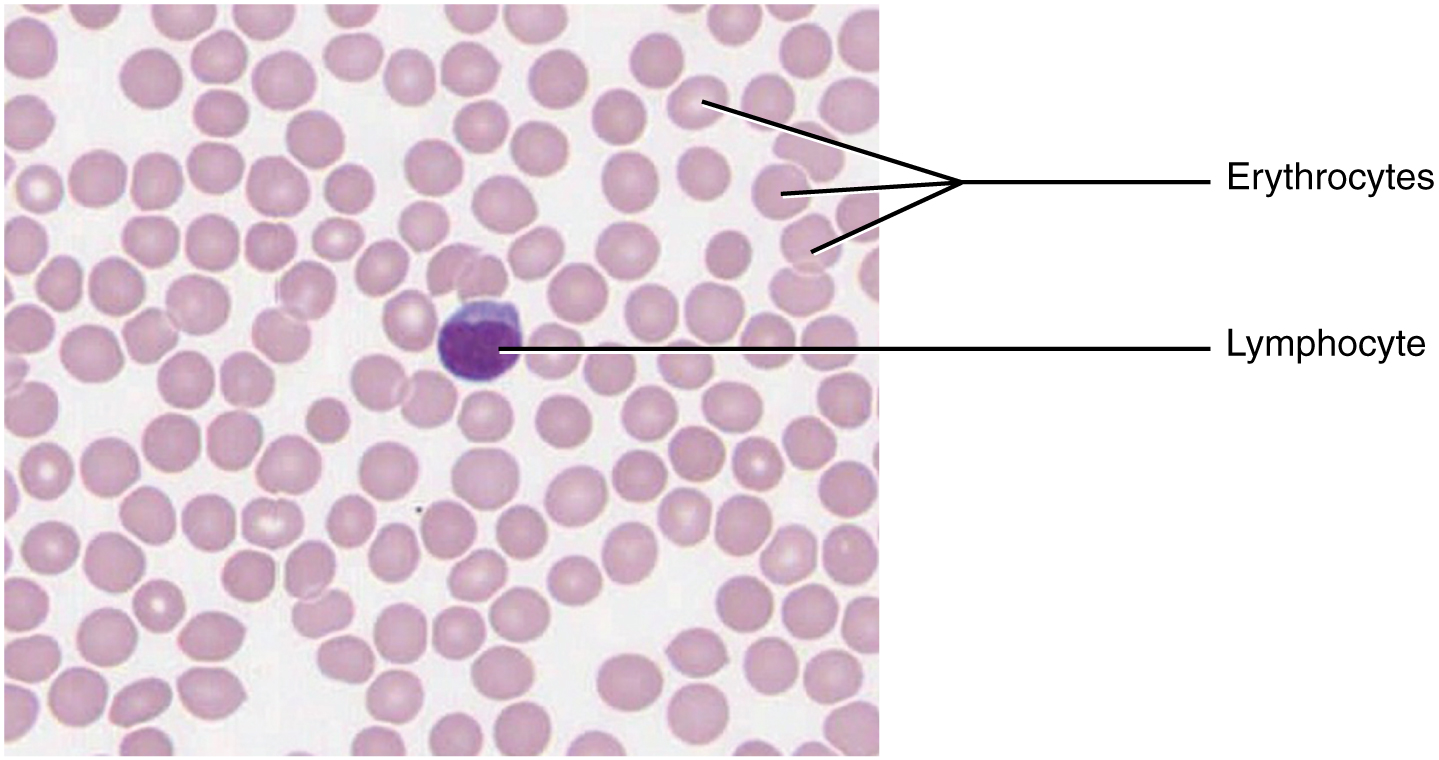
Blood and lymph are fluid connective tissues. Cells circulate in a liquid extracellular matrix. The formed elements circulating in the blood are derived from hematopoietic stem cells located in the bone marrow and include red blood cells (RBCs), white blood cells (WBCs), and cell fragments called platelets. Erythrocytes, red blood cells, transport oxygen, and some carbon dioxide. Leukocytes, white blood cells, are responsible for defending against potentially harmful microorganisms or molecules. Platelets are cell fragments involved in blood clotting. Some white blood cells have the ability to cross the endothelial layer that lines blood vessels and enter adjacent tissues. The extracellular matrix, called plasma, makes blood unique among connective tissues because it is fluid. This fluid, which is mostly water, perpetually suspends the formed elements and enables them to circulate throughout the body within the cardiovascular system. Nutrients, salts, and wastes are dissolved in the liquid matrix and transported through the body. Blood constitutes approximately 8 percent of adult body weight. Adult males typically average about 5-6 liters of blood. Females average 4–5 liters.
Functions of Blood
The primary function of blood is to deliver oxygen and nutrients to and remove wastes from body cells, but that is only the beginning of the story. The specific functions of blood also include defense and regulation.
Transportation
- Oxygen and carbon dioxide from the air you breathe and cellular metabolism.
- Nutrients from the foods you eat are absorbed in the digestive tract. Most of these travel in the bloodstream directly to the liver, where they are processed and released back into the bloodstream for delivery to body cells.
- Hormones are released by endocrine glands into the bloodstream.
- Enzymes and waste products are transported from cells in the bloodstream.
Defense
Many types of WBCs protect the body from external threats, such as disease-causing bacteria that have entered the bloodstream in a wound. Other WBCs seek out and destroy internal threats, such as cells with mutated DNA that could multiply to become cancerous or body cells infected with viruses.
When damage to the vessels results in bleeding, blood platelets and certain proteins dissolved in the plasma, the fluid portion of the blood, interact to block the ruptured areas of the blood vessels involved. This protects the body from further blood loss.
Maintenance of Homeostasis
- Chemical balance. Proteins and other compounds in the blood act as buffers, thereby regulating the pH of body tissues. Blood also helps to regulate the water and electrolyte content of body cells.
- Body temperature. Blood can be transported to the integument to be cooled and thereby cooling the body’s core upon return. In contrast, with extreme cold, blood can be transported away from the integument to maintain a warmer body core.
Hematopoiesis (hemopoiesis)
Before birth, hematopoiesis occurs in a number of tissues, beginning with the yolk sac of the developing embryo, and continuing in the fetal liver, spleen, lymphatic tissue, and eventually the red bone marrow. Following birth, most hemopoiesis occurs in the red marrow, a connective tissue within the spaces of spongy (cancellous) bone tissue. In children, hemopoiesis can occur in the medullary cavity of long bones; in adults, the process is largely restricted to the cranial and pelvic bones, the vertebrae, sternum, and the proximal epiphyses of the femur and humerus.
Throughout adulthood, the liver and spleen maintain their ability to generate the formed elements. This process is referred to as extramedullary hemopoiesis (meaning hemopoiesis outside the medullary cavity of adult bones). When a disease such as bone cancer destroys the bone marrow, causing hemopoiesis to fail, extramedullary hemopoiesis may be initiated.
The lifespan of the formed elements is very brief. Although one type of leukocyte called memory cells can survive for years, most erythrocytes, leukocytes, and platelets normally live only a few hours to a few weeks. Thus, the body must form new blood cells and platelets quickly and continuously. When you donate a unit of blood during a blood drive (approximately 475 mL, or about 1 pint), your body typically replaces the donated plasma within 24 hours, but it takes about 4 to 6 weeks to replace the blood cells. This restricts the frequency with which donors can contribute their blood. The process by which this replacement occurs is called hemopoiesis, or hematopoiesis (from the Greek root haima- = “blood”; -poiesis = “production”).
Differentiation of Formed Elements from Stem Cells
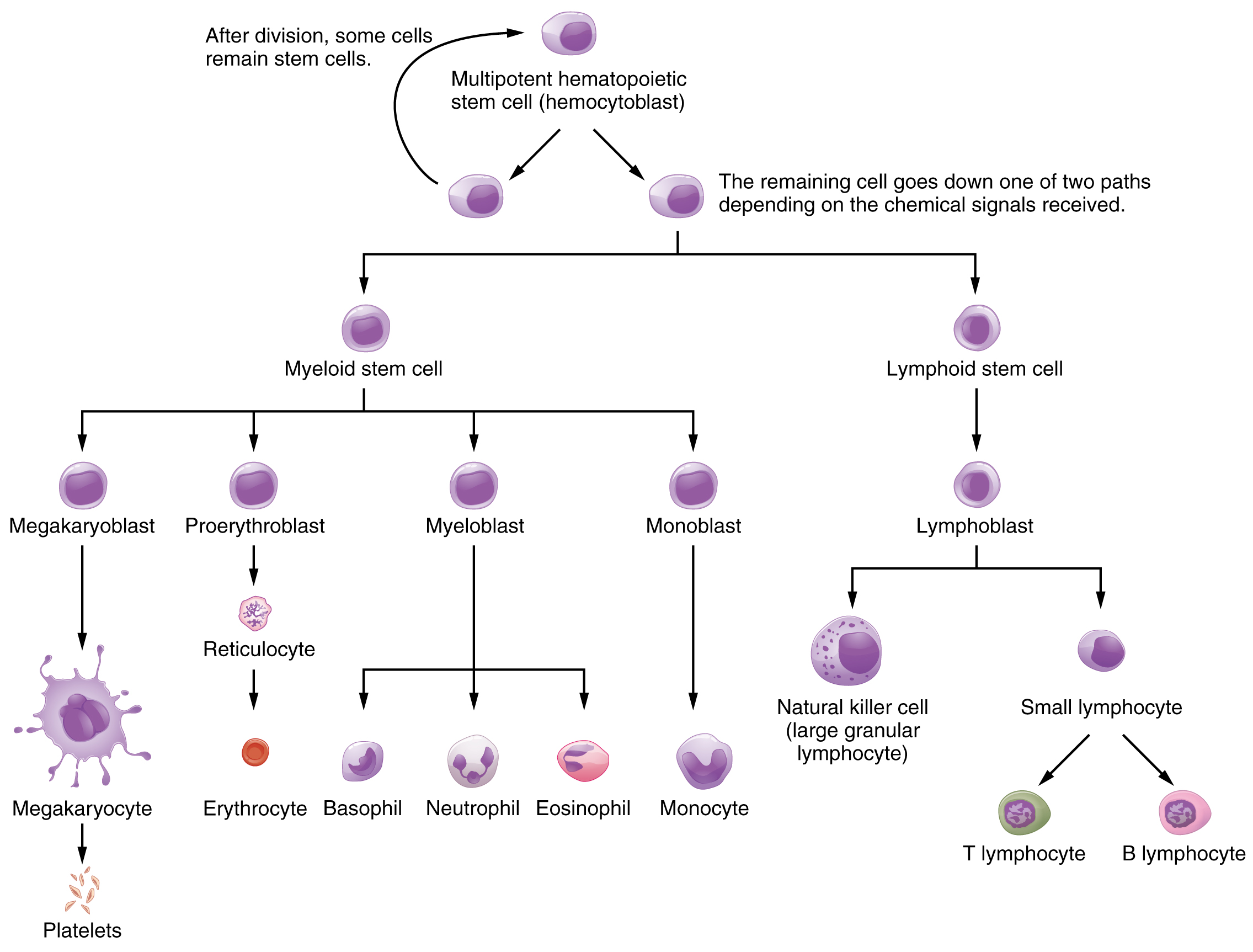
All formed elements arise from stem cells of the red bone marrow. Recall that stem cells undergo mitosis plus cytokinesis (cellular division) to give rise to new daughter cells: one of these remains a stem cell. The other differentiates into one of any number of diverse cell types. Stem cells may be viewed as occupying a hierarchal system, with some loss of the ability to diversify at each step.
Hematopoiesis begins when the hemopoietic stem cell is exposed to appropriate chemical stimuli collectively called hemopoietic growth factors, which prompt it to divide and differentiate. One daughter cell remains a hemopoietic stem cell, allowing hemopoiesis to continue. The other daughter cell becomes either of two types of more specialized stem cells:
- Lymphoid stem cells give rise to a class of leukocytes known as lymphocytes, including the various T cells, B cells, and natural killer (NK) cells, all of which function in immunity. However, hemopoiesis of lymphocytes progresses somewhat differently from the process for the other formed elements. In brief, lymphoid stem cells quickly migrate from the bone marrow to lymphatic tissues, including the lymph nodes, spleen, and thymus, where their production and differentiation continues. B cells are so named since they mature in the bone marrow, while T cells mature in the thymus.
- Myeloid stem cells give rise to all the other formed elements, including the erythrocytes; megakaryocytes that produce platelets; and a myeloblast lineage that gives rise to monocytes and three forms of granular leukocytes: neutrophils, eosinophils, and basophils.
Composition of Blood
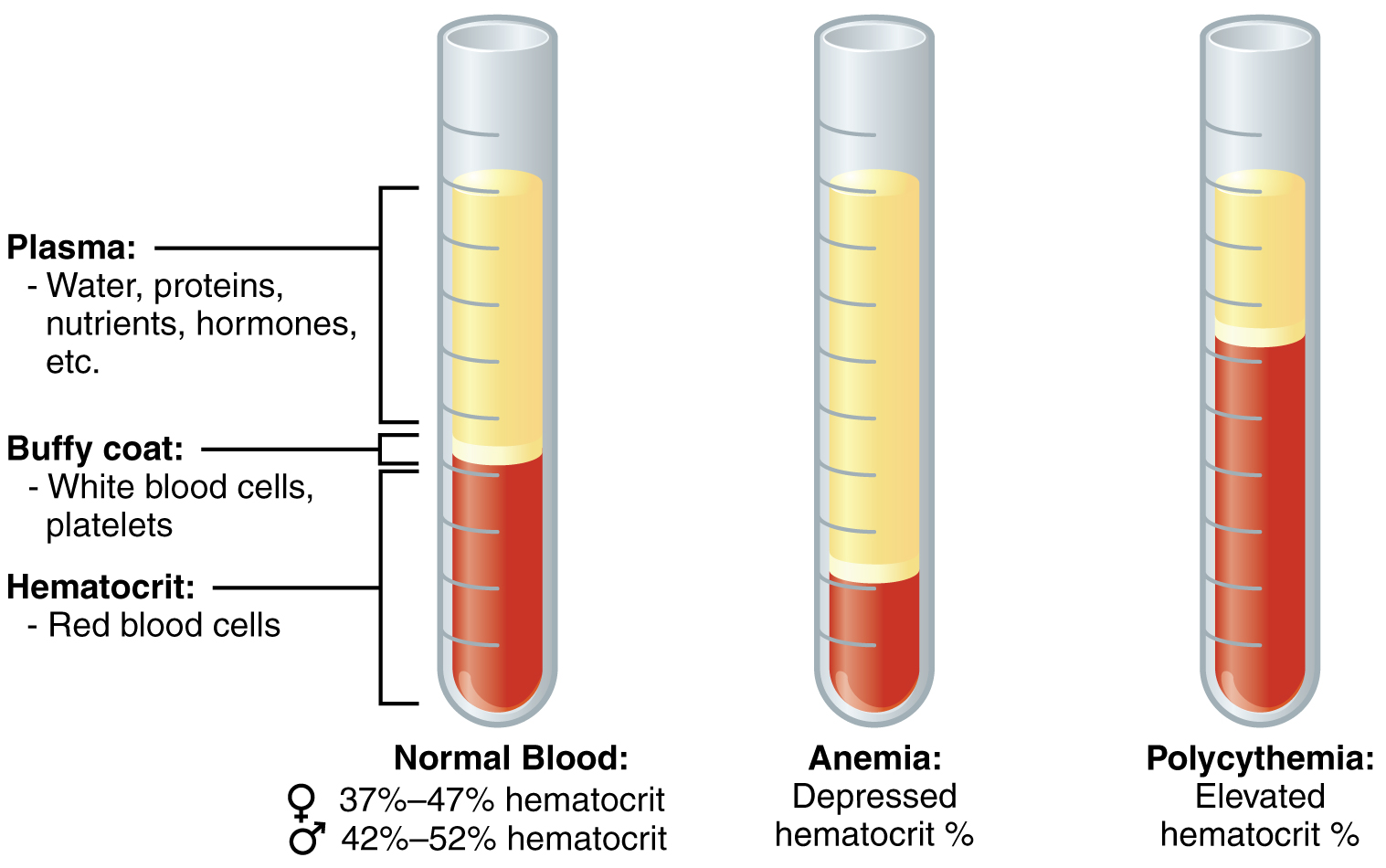
We can see the components of blood using a test called a hematocrit, which measures the percentage of RBCs, clinically known as erythrocytes, in a blood sample. It is performed by spinning the blood sample in a specialized centrifuge, a process that causes the heavier elements suspended within the blood sample to separate from the lightweight, liquid plasma. Because the heaviest elements in blood are the erythrocytes, these settle at the very bottom of the hematocrit tube. Located above the erythrocytes is a pale, thin layer composed of the remaining formed elements of blood. These are the WBCs, clinically known as leukocytes, and the platelets, cell fragments also called thrombocytes. This layer is referred to as the buffy coat because of its color; it normally constitutes less than 1 percent of a blood sample. Above the buffy coat is the blood plasma, normally a pale, straw-colored fluid, which constitutes the remainder of the sample.
In normal blood, about 45 percent of a sample is erythrocytes. The hematocrit of any one sample can vary significantly, however, about 36–50 percent, according to gender and other factors. The percentage of other formed elements, the WBCs, and platelets, is tiny, ~1%. So the plasma percentage is the percent of the blood that is not erythrocytes and is about 55%.
Blood Plasma
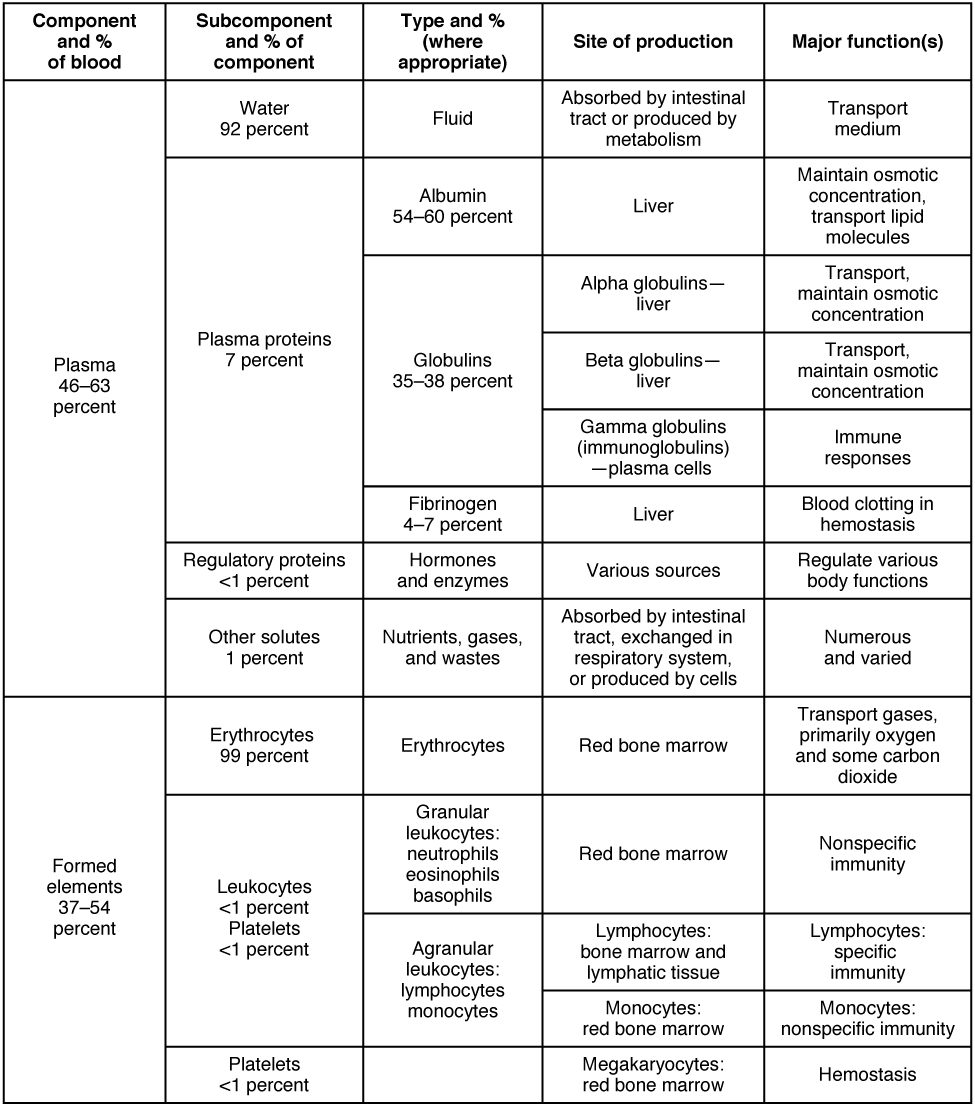
Like other fluids in the body, plasma is composed primarily of water: It is about 92 percent water. Dissolved or suspended within this water is a mixture of substances, most of which are proteins. There are literally hundreds of substances dissolved or suspended in the plasma, although many are found only in tiny quantities.
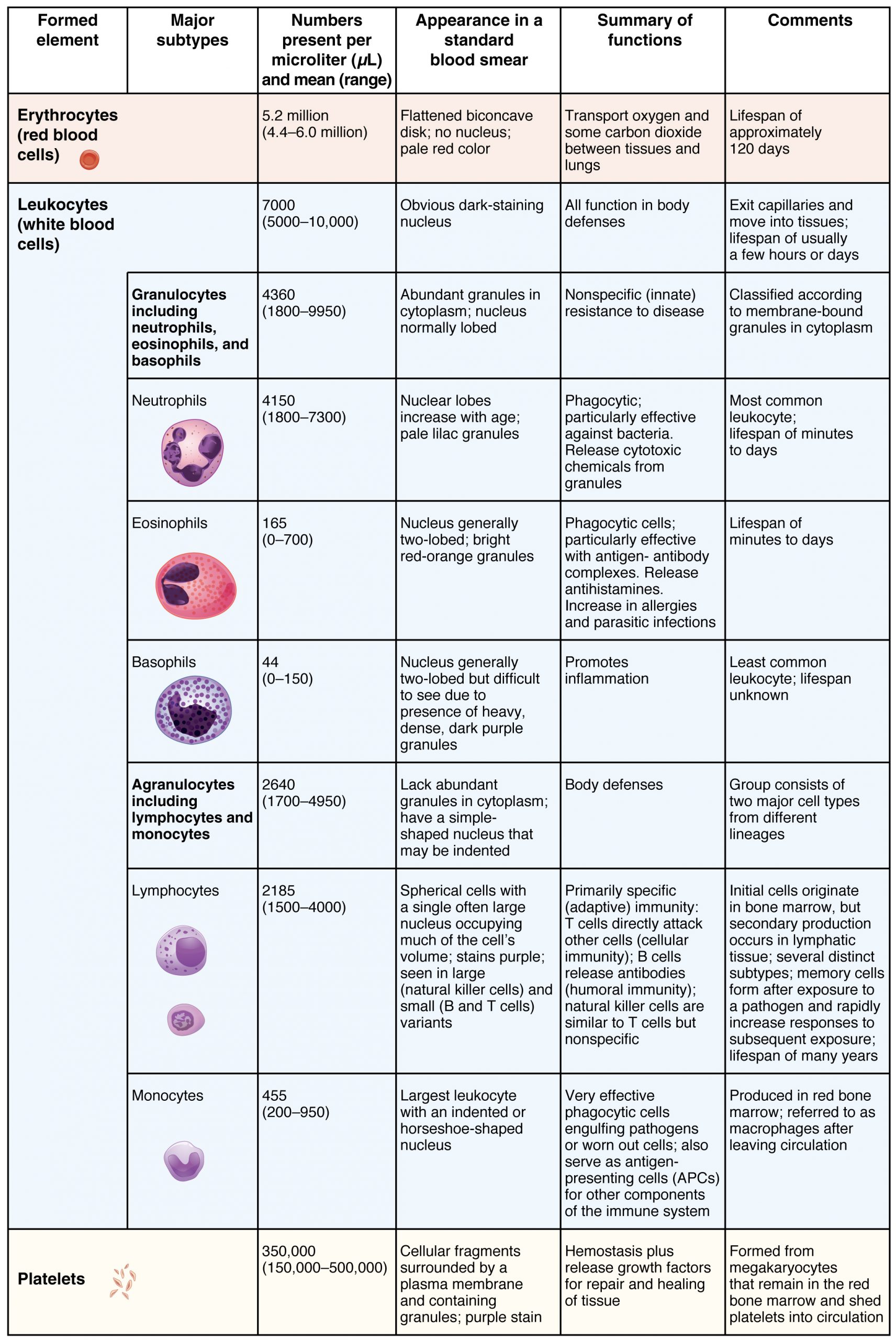
Erythrocytes: Red Blood Cells
The erythrocyte, commonly known as a red blood cell (or RBC), is by far the most common formed element: a single drop of blood contains millions of erythrocytes and just thousands of leukocytes. In fact, erythrocytes are estimated to make up about 25 percent of the total cells in the body. As you can imagine, they are quite small cells, with a mean diameter of only about 7–8 micrometers (µm). Erythrocytes’ primary functions are to pick up inhaled oxygen from the lungs and transport it to the body’s tissues and pick up some (about 24 percent) carbon dioxide waste at the tissues and transport it to the lungs for exhalation. Erythrocytes live up to 120 days in circulation, after which the worn-out cells are removed by a type of myeloid phagocytic cell called a macrophage, located primarily within the bone marrow, liver, and spleen. Erythrocytes remain within the vascular network. Although leukocytes typically leave the blood vessels to perform their defensive functions, the movement of erythrocytes from the blood vessels is abnormal.
Erythrocytes are biconcave disks; they are plump at their periphery and very thin in the center. Since they lack most organelles, there is more interior space for the presence of the hemoglobin molecules that, as you will see shortly, transport gases. The biconcave shape also provides a greater surface area across which gas exchange can occur, relative to its volume; a sphere of a similar diameter would have a lower surface area-to-volume ratio.
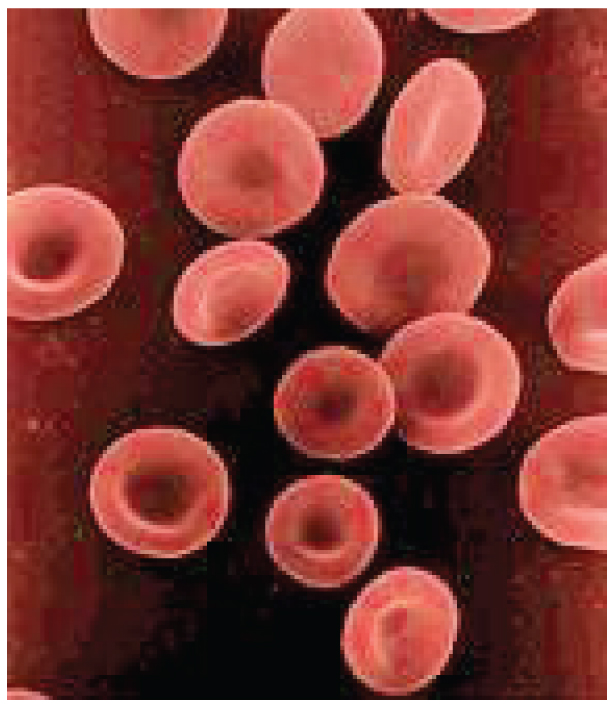
Hemoglobin
Hemoglobin is a large molecule made up of proteins and iron. It consists of four folded chains of a protein called globin, designated alpha 1 and 2, and beta 1 and 2. These globin molecules are bound to a red pigment molecule called heme, which contains an ion of iron (Fe2+).
RBCs – FYI (For Your Information -> you will not be assessed on this content)
Each iron ion in the heme can bind to one oxygen molecule; therefore, each hemoglobin molecule can transport four oxygen molecules. An individual erythrocyte may contain about 300 million hemoglobin molecules, and therefore can bind to and transport up to 1.2 billion oxygen molecules.
In the lungs, hemoglobin picks up oxygen, which binds to the iron ions, forming oxyhemoglobin. The bright red, oxygenated hemoglobin travels to the body tissues, where it releases some of the oxygen molecules, becoming darker red deoxyhemoglobin, sometimes referred to as reduced hemoglobin. Oxygen release depends on the need for oxygen in the surrounding tissues, so hemoglobin rarely, if ever, leaves all of its oxygen behind. In the capillaries, carbon dioxide enters the bloodstream. About 76 percent dissolves in the plasma, some of it remaining as dissolved CO2 and the remainder forming bicarbonate ion. About 23–24 percent binds to the amino acids in hemoglobin, forming a molecule known as carbaminohemoglobin. From the capillaries, the hemoglobin carries carbon dioxide back to the lungs, where it releases it for the exchange of oxygen.
Changes in RBCs levels can have significant effects on the body’s ability to effectively deliver oxygen to the tissues. Ineffective hematopoiesis results in insufficient numbers of RBCs and results in one of several forms of anemia. In patients with insufficient hemoglobin, the tissues may not receive sufficient oxygen, resulting in another form of anemia. In determining the oxygenation of tissues, the value of the greatest interest in healthcare is the percent saturation; that is, the percentage of hemoglobin sites occupied by oxygen in a patient’s blood. Clinically this value is commonly referred to simply as “percent sat.”
Percent saturation is normally monitored using a pulse oximeter device, which is applied to a thin part of the body, typically the tip of the patient’s finger. The device works by sending two different wavelengths of light (one red, the other infrared) through the finger and measuring the light with a photodetector as it exits. Hemoglobin absorbs light differentially depending upon its saturation with oxygen. The machine calibrates the amount of light received by the photodetector against the amount absorbed by the partially oxygenated hemoglobin and presents the data as percent saturation. Normal pulse oximeter readings range from 95–100 percent. Lower percentages reflect hypoxemia or low blood oxygen. The term hypoxia is more generic and simply refers to low oxygen levels. Oxygen levels are also directly monitored from free oxygen in the plasma, typically following an arterial stick. When this method is applied, the amount of oxygen present is expressed in terms of the partial pressure of oxygen or simply pO2 and is typically recorded in units of millimeters of mercury, mm Hg.
Disorders of Erythrocytes
The size, shape, and number of erythrocytes and the number of hemoglobin molecules can significantly impact a person’s health. When the number of RBCs or hemoglobin is deficient, the general condition is called anemia. There are more than 400 types of anemia, and more than 3.5 million Americans suffer from this condition. Anemia can be broken down into three major groups: those caused by blood loss, those caused by faulty or decreased RBC production, and those caused by excessive destruction of RBCs.
Blood loss anemias are fairly straightforward. In addition to bleeding from wounds or other lesions, these forms of anemia may be due to ulcers, hemorrhoids, inflammation of the stomach (gastritis), and some cancers of the gastrointestinal tract. The excessive use of aspirin or other nonsteroidal anti-inflammatory drugs such as ibuprofen can trigger ulceration and gastritis. Excessive menstruation and loss of blood during childbirth are also potential causes.
Anemias caused by faulty or decreased RBC production include sickle cell anemia, iron deficiency anemia, vitamin deficiency anemia, and diseases of the bone marrow and stem cells.
- A characteristic change in the shape of erythrocytes is seen in sickle cell disease (also referred to as sickle cell anemia). A genetic disorder, it is caused by the production of an abnormal type of hemoglobin, called hemoglobin S, which delivers less oxygen to tissues and causes erythrocytes to assume a sickle (or crescent) shape, especially at low oxygen concentrations. These abnormally shaped cells can then become lodged in narrow capillaries because they cannot fold in on themselves to squeeze through, blocking blood flow to tissues and causing a variety of serious problems from painful joints to delayed growth and even blindness and cerebrovascular accidents (strokes). Sickle cell anemia is a genetic condition particularly found in individuals of African descent.
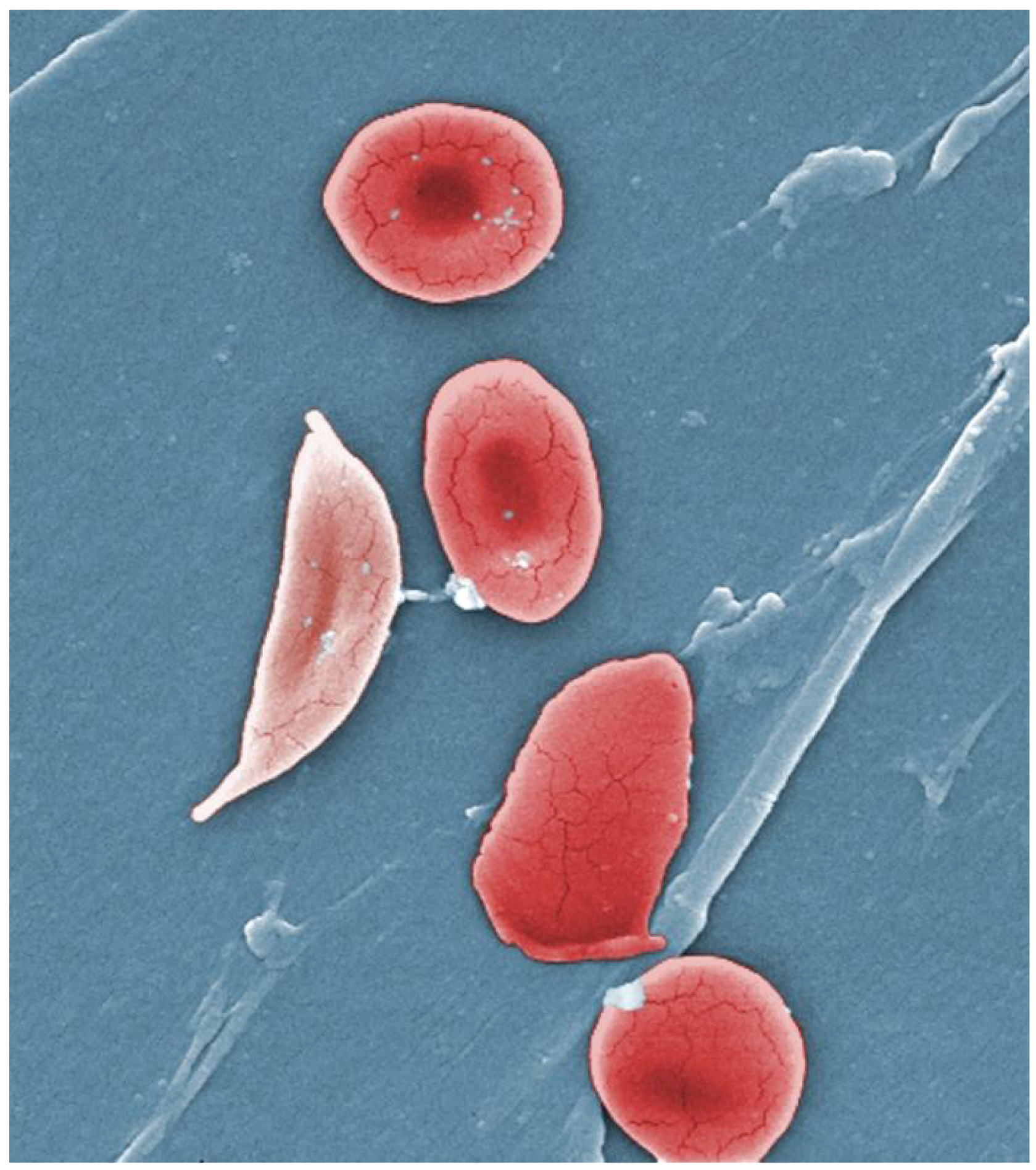
- Iron deficiency anemia is the most common type and results when the amount of available iron is insufficient to allow the production of sufficient heme. This condition can occur in individuals with a deficiency of iron in the diet and is especially common in teens and children and vegans and vegetarians. Additionally, iron deficiency anemia may be caused by either an inability to absorb and transport iron or slow, chronic bleeding.
Vitamin-deficient anemias – FYI (For Your Information -> you will not be assessed on this content)
Vitamin-deficient anemias generally involve insufficient vitamin B12 and folate.
- Megaloblastic anemia involves a deficiency of vitamin B12 and/or folate and often involves diets deficient in these essential nutrients. Lack of meat or a viable alternate source and overcooking or eating insufficient amounts of vegetables may lead to a lack of folate.
- Pernicious anemia is caused by poor absorption of vitamin B12. It is often seen in patients with Crohn’s disease (a severe intestinal disorder often treated by surgery), surgical removal of the intestines or stomach (common in some weight-loss surgeries), intestinal parasites, and AIDS.
- Pregnancies, some medications, excessive alcohol consumption, and some diseases such as celiac disease are also associated with vitamin deficiencies. It is essential to provide sufficient folic acid during the early stages of pregnancy to reduce the risk of neurological defects, including spina bifida, a failure of the neural tube to close.
Disease processes leading to anemia – FYI (For Your Information > you will not be assessed on this content)
Assorted disease processes can also interfere with the production and formation of RBCs and hemoglobin. If myeloid stem cells are defective or replaced by cancer cells, there will be insufficient RBCs produced.
- Aplastic anemia is a condition in which there are deficient numbers of RBC stem cells. Aplastic anemia is often inherited, or it may be triggered by radiation, medication, chemotherapy, or infection.
- Thalassemia is an inherited condition typically occurring in individuals from the Middle East, the Mediterranean, Africa, and Southeast Asia, in which maturation of the RBCs do not proceed normally. The most severe form is called Cooley’s anemia.
- Lead exposure from industrial sources or even dust from paint chips of iron-containing paints or pottery that has not been properly glazed may also lead to the destruction of the red marrow.
- Various disease processes also can lead to anemias. These include chronic kidney diseases often associated with a decreased production of EPO, hypothyroidism, some forms of cancer, lupus, and rheumatoid arthritis.
Blood Types
Although the ABO blood group name consists of three letters, ABO blood typing designates the presence or absence of just two antigens, A and B. Both are glycoproteins. People whose erythrocytes have A antigens on their erythrocyte membrane surfaces are designated blood type A, and those whose erythrocytes have B antigens are blood type B. People can also have both A and B antigens on their erythrocytes, in which case they are blood type AB. People with neither A nor B antigens are designated blood type O. ABO blood types are genetically determined.
Normally the body must be exposed to a foreign antigen before an antibody can be produced. This is not the case for the ABO blood group. Individuals with type A blood—without any prior exposure to incompatible blood—have preformed antibodies to the B antigen circulating in their blood plasma. These antibodies, referred to as anti-B antibodies, will cause agglutination and hemolysis if they encounter erythrocytes with B antigens. Similarly, an individual with type B blood has pre-formed anti-A antibodies. Individuals with type AB blood, which has both antigens, do not have preformed antibodies to either of these. People with type O blood lack antigens A and B on their erythrocytes, but anti-A and anti-B antibodies circulate in their blood plasma.
Determining ABO Blood Types – FYI (For Your Information -> you will not be assessed on this content)
Clinicians can determine a patient’s blood type quickly and easily using commercially prepared antibodies. An unknown blood sample is allocated into separate wells. Into one well, a small amount of anti-A antibody is added, and in another, a small amount of anti-B antibody. If the antigen is present, the antibodies will cause visible agglutination of the cells. The blood should also be tested for Rh antibodies.
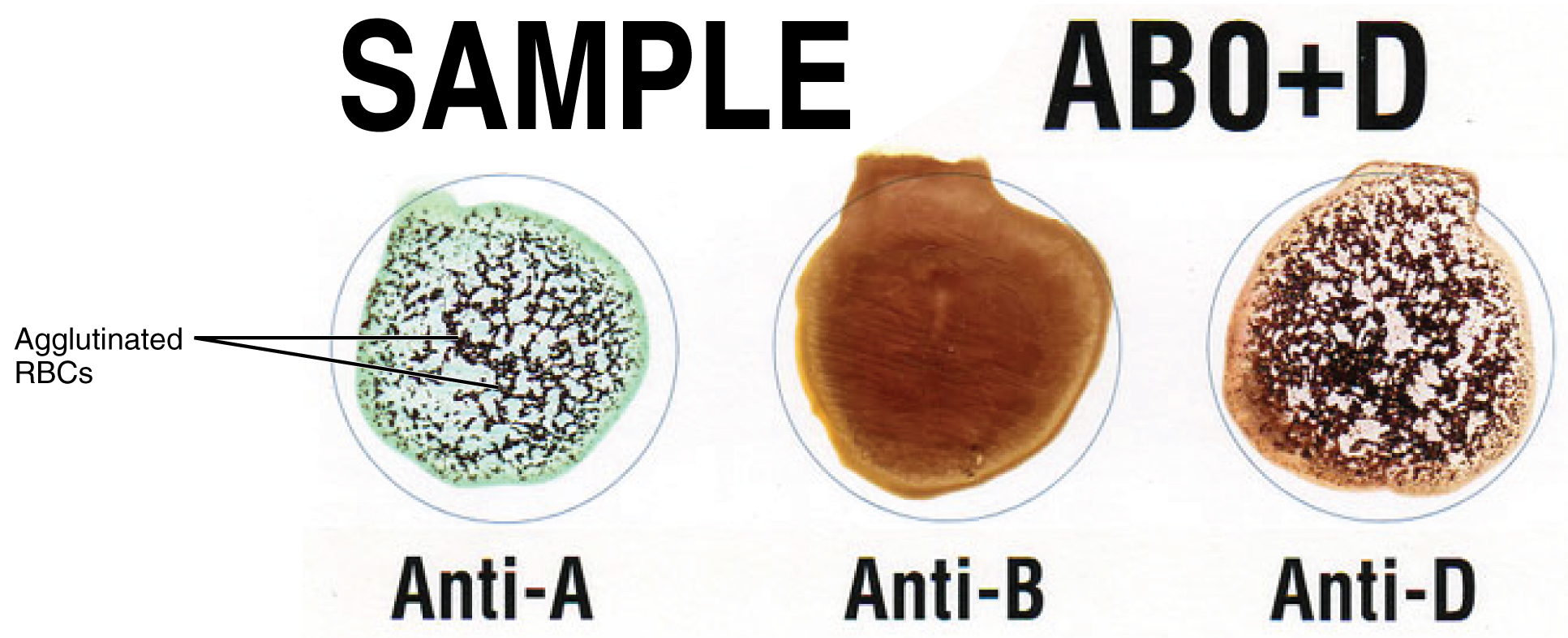
Rh Blood Groups
The Rh blood group is classified according to the presence or absence of a second erythrocyte antigen identified as Rh. Although dozens of Rh antigens have been identified, only one, designated D, is clinically important. Those who have the Rh D antigen present on their erythrocytes—about 85 percent of Americans—are described as Rh positive (Rh+), and those who lack it are Rh-negative (Rh−). Note that the Rh group is distinct from the ABO group, so any individual, no matter their ABO blood type, may have or lack this Rh antigen. When identifying a patient’s blood type, the Rh group is designated by adding the word positive or negative to the ABO type. For example, A positive (A+) means ABO group A blood with the Rh antigen present, and AB negative (AB−) means ABO group AB blood without the Rh antigen.
Fun Fact – ABO and Rh Blood Types in the U.S. (-> you will not be assessed on this content)
| Summary of ABO and Rh Blood Types within the United States | ||||
|---|---|---|---|---|
| Blood Type | African-Americans | Asian-Americans | Caucasian-Americans | Latino/Latina-Americans |
| A+ | 24 | 27 | 33 | 29 |
| A− | 2 | 0.5 | 7 | 2 |
| B+ | 18 | 25 | 9 | 9 |
| B− | 1 | 0.4 | 2 | 1 |
| AB+ | 4 | 7 | 3 | 2 |
| AB− | 0.3 | 0.1 | 1 | 0.2 |
| O+ | 47 | 39 | 37 | 53 |
| O− | 4 | 1 | 8 | 4 |
In contrast to the ABO group antibodies, which are preformed, antibodies to the Rh antigen are produced only in Rh− individuals after exposure to the antigen. This process, called sensitization, occurs following a transfusion with Rh-incompatible blood or, more commonly, with the birth of an Rh+ baby to an Rh− mother. Problems are rare in a first pregnancy since the baby’s Rh+ cells rarely cross the placenta (the organ of gas and nutrient exchange between the baby and the mother). However, during or immediately after birth, the Rh− mother can be exposed to the baby’s Rh+ cells. Research has shown that this occurs in about 13−14 percent of such pregnancies. After exposure, the mother’s immune system begins to generate anti-Rh antibodies. If the mother should then conceive another Rh+ baby, the Rh antibodies she has produced can cross the placenta into the fetal bloodstream and destroy the fetal RBCs. This condition, known as hemolytic disease of the newborn (HDN) or erythroblastosis fetalis, may cause anemia in mild cases. Still, the agglutination and hemolysis can be so severe that the fetus may die in the womb or shortly after birth without treatment.
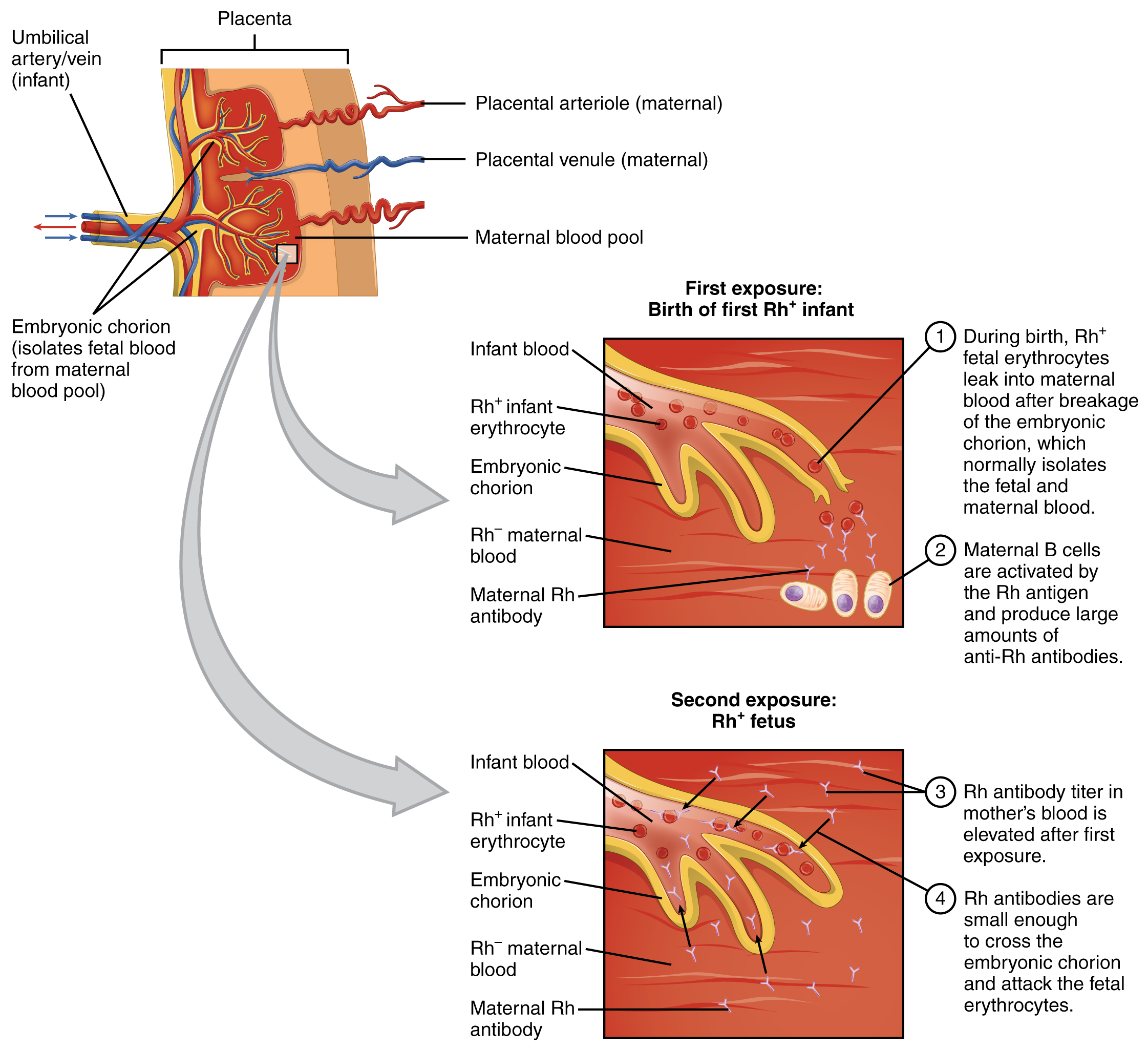
Fun Fact – Rh immune globulin (-> you will not be assessed on this content)
A drug known as RhoGAM, short for Rh immune globulin, can temporarily prevent the development of Rh antibodies in the Rh− mother, thereby averting this potentially serious disease for the fetus. RhoGAM antibodies destroy any fetal Rh+ erythrocytes that may cross the placental barrier. RhoGAM is normally administered to Rh− mothers during weeks 26−28 of pregnancy and within 72 hours following birth. It has proven remarkably effective in decreasing the incidence of HDN. Earlier, we noted that the incidence of HDN in an Rh+ subsequent pregnancy to an Rh− mother is about 13–14 percent without preventive treatment. Since the introduction of RhoGAM in 1968, the incidence has dropped to about 0.1 percent in the United States.
ABO Transfusion Protocols
To avoid transfusion reactions, it is best to transfuse only matching blood types; that is, a type B+ recipient should ideally receive blood only from a type B+ donor and so on. That said, in emergencies, when acute hemorrhage threatens the patient’s life, there may not be time for cross-matching to identify blood type. In these cases, blood from a universal donor—an individual with type O− blood—may be transfused. Recall that type O erythrocytes do not display A or B antigens. Thus, anti-A or anti-B antibodies that might be circulating in the patient’s blood plasma will not encounter any erythrocyte surface antigens on the donated blood and therefore will not be provoked into a response. One problem with this designation of the universal donor is if the O− individual had prior exposure to Rh antigen, Rh antibodies might be present in the donated blood. Also, introducing type O blood into an individual with type A, B, or AB blood will introduce antibodies against both A and B antigens, as these are always circulating in the type O blood plasma. This may cause problems for the recipient, but because the volume of blood transfused is much lower than the volume of the patient’s own blood, the adverse effects of the relatively few infused plasma antibodies are typically limited. Rh factor also plays a role. If Rh− individuals receiving blood have had prior exposure to Rh antigen, antibodies for this antigen may be present in the blood and trigger agglutination to some degree. Although it is always preferable to cross-match a patient’s blood before transfusing, this is not always possible in a true life-threatening emergency, and these procedures may be implemented.
A patient with blood type AB+ is known as the universal recipient. This patient can theoretically receive any type of blood because the patient’s own blood—having both A and B antigens on the erythrocyte surface—does not produce anti-A or anti-B antibodies. In addition, an Rh+ patient can receive both Rh+ and Rh− blood. However, keep in mind that the donor’s blood will contain circulating antibodies, again with possible negative implications.
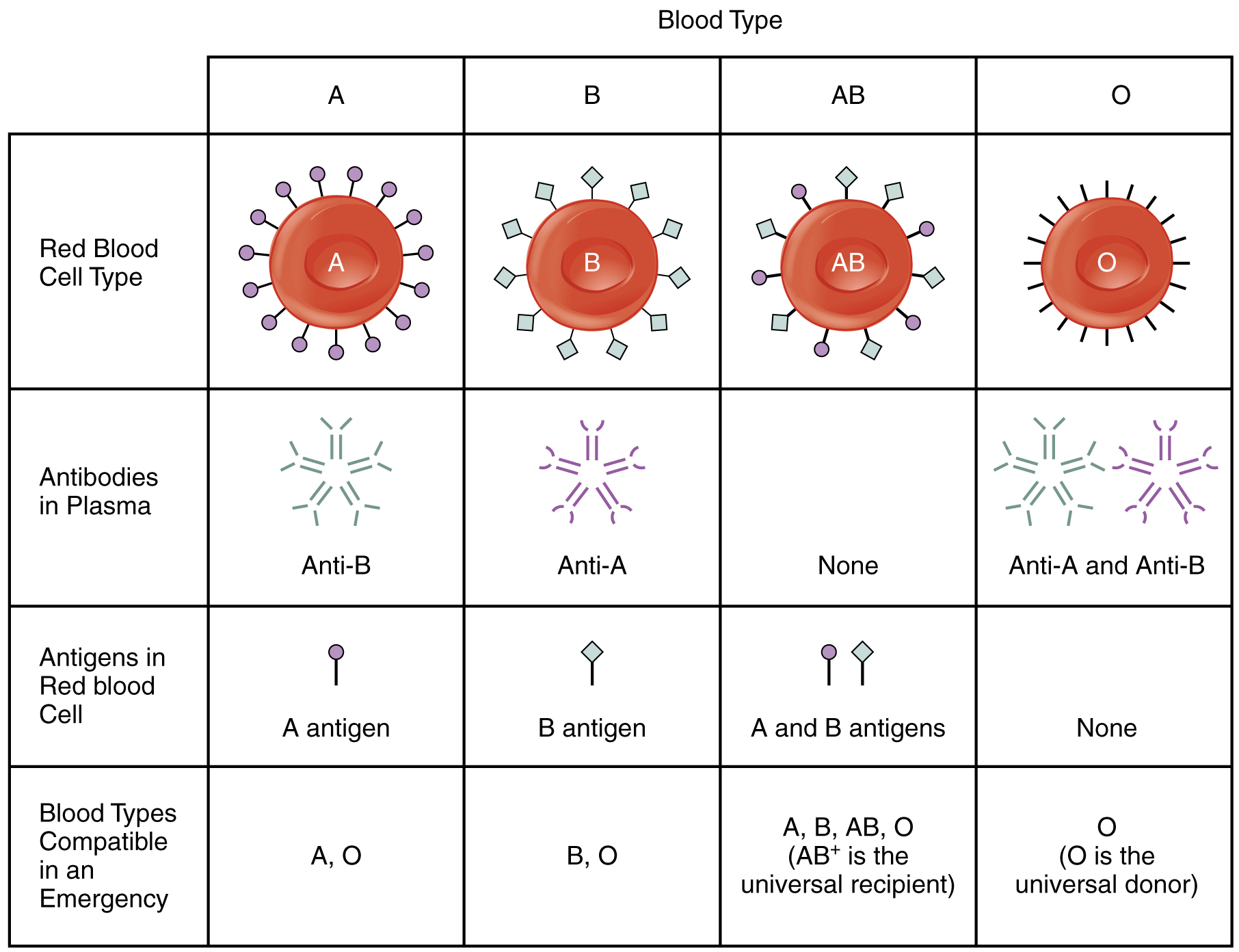
Leukocytes: White Blood Cells
The leukocyte, commonly known as a white blood cell (or WBC), is a major component of the body’s defenses against disease. Leukocytes protect the body against invading microorganisms and body cells with mutated DNA, and they clean up debris. Platelets are essential for repairing blood vessels when damage to them has occurred; they also provide growth factors for healing and repair.
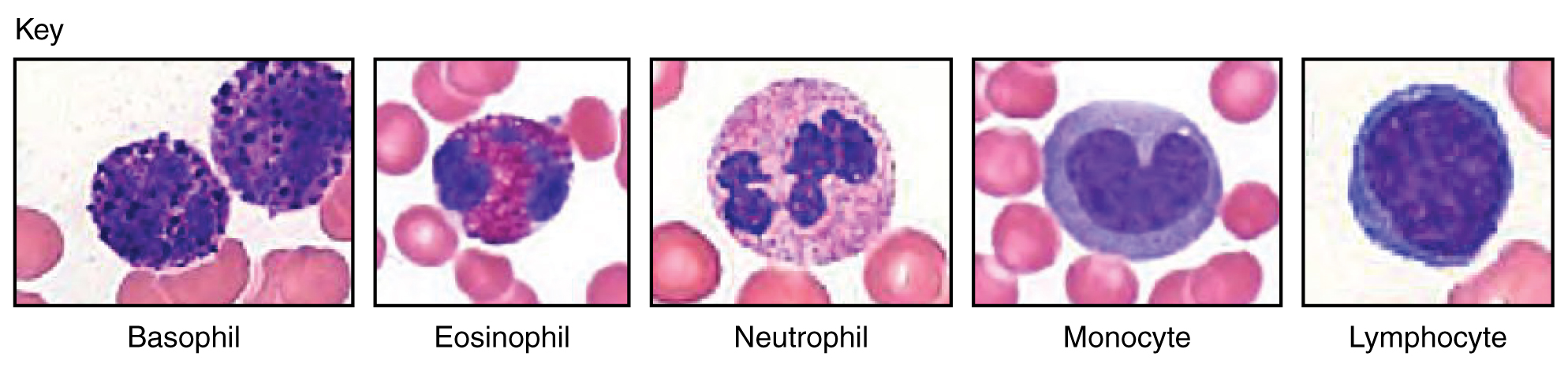
Characteristics of Leukocytes
Although leukocytes and erythrocytes both originate from hematopoietic stem cells in the bone marrow, they differ in many significant ways. For instance, leukocytes are far less numerous than erythrocytes: Typically, there are only 5000 to 10,000 per µL. They are also larger than erythrocytes and are the only formed elements that are complete cells, possessing a nucleus and organelles. And although there is just one type of erythrocyte, there are many types of leukocytes. Most of these types have a much shorter lifespan than that of erythrocytes, some as short as a few hours or even a few minutes in the case of acute infection.
One of the most distinctive characteristics of leukocytes is their movement. Whereas erythrocytes spend their days circulating within the blood vessels, leukocytes routinely leave the bloodstream to perform their defensive functions in the body’s tissues. For leukocytes, the vascular network is simply a highway they travel and soon exit to reach their true destination. When they arrive, they are often given distinct names, such as macrophage or microglia, depending on their function.
Once they have exited the capillaries, some leukocytes will take up fixed positions in lymphatic tissue, bone marrow, the spleen, the thymus, or other organs. Others will move about through the tissue spaces very much like amoebas, continuously extending their plasma membranes, sometimes wandering freely, and sometimes moving toward the direction they are drawn by chemical signals.
Emigration – FYI (For Your Information -> you will not be assessed on this content)
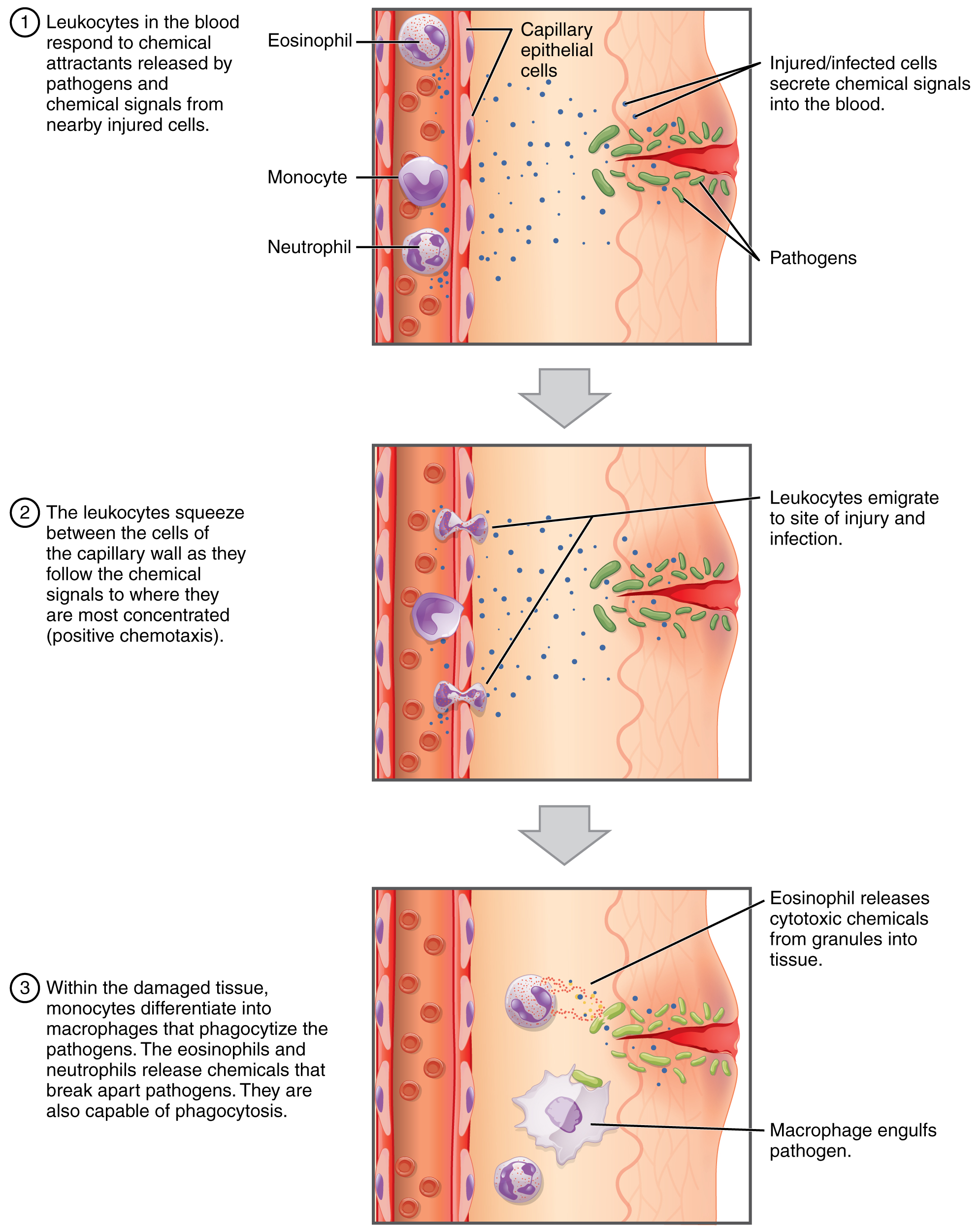
Classification of Leukocytes
When scientists first began to observe stained blood slides, it quickly became evident that leukocytes could be divided into two groups, according to whether their cytoplasm contained highly visible granules:
- Granular leukocytes contain abundant granules within the cytoplasm. They include neutrophils, eosinophils, and basophils.
- While granules are not totally lacking in agranular leukocytes, they are far fewer and less obvious. Agranular leukocytes include monocytes, which mature into macrophages that are phagocytic, and lymphocytes, which arise from the lymphoid stem cell line.
Granular Leukocytes
We will consider the granular leukocytes in order from most common to least common. All of these are produced in the red bone marrow and have a short lifespan of hours to days. They typically have a lobed nucleus and are classified according to which type of stain best highlights their granules.
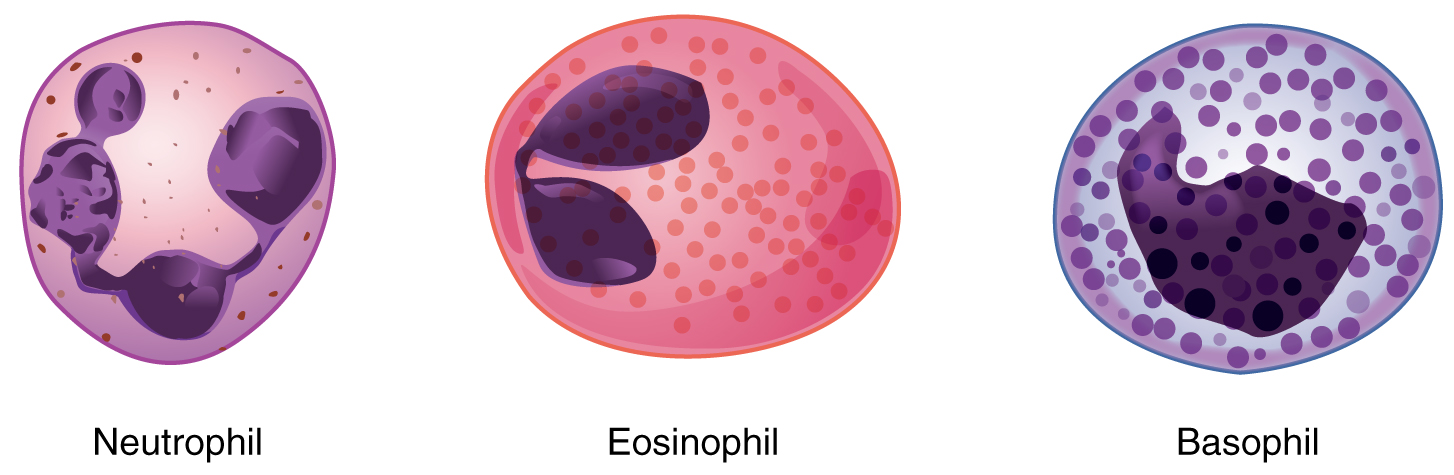
Neutrophils
The most common of all the leukocytes, neutrophils will normally comprise 50–70 percent of the total leukocyte count. They are 10–12 µm in diameter, significantly larger than erythrocytes. They are called neutrophils because their granules show up most clearly with stains that are chemically neutral (neither acidic nor basic).
Neutrophils are rapid responders to the site of infection and are efficient phagocytes with a preference for bacteria. Their granules include lysozyme, an enzyme capable of lysing or breaking down bacterial cell walls; oxidants such as hydrogen peroxide; and defensins, proteins that bind to and puncture bacterial and fungal plasma membranes so that the cell contents leak out. Abnormally high counts of neutrophils indicate infection and/or inflammation, particularly triggered by bacteria, but are also found in burn patients and others experiencing unusual stress. A burn injury increases the proliferation of neutrophils to fight off an infection that can result from the destruction of the barrier of the skin. Low counts may be caused by drug toxicity and other disorders and may increase an individual’s susceptibility to infection.
Eosinophils
Eosinophils typically represent 2–4 percent of the total leukocyte count. They are also 10–12 µm in diameter. The granules of eosinophils stain best with an acidic stain known as eosin. The granules of eosinophils include antihistamine molecules, which counteract the activities of histamines, inflammatory chemicals produced by basophils, and mast cells. Some eosinophil granules contain molecules toxic to parasitic worms, which can enter the body through the integument, or when an individual consumes raw or undercooked fish or meat. Eosinophils are also capable of phagocytosis and are particularly effective when antibodies bind to the target and form an antigen-antibody complex. High counts of eosinophils are typical of patients experiencing allergies, parasitic worm infestations, and some autoimmune diseases. Low counts may be due to drug toxicity and stress.
Basophils
Basophils are the least common leukocytes, typically comprising less than one percent of the total leukocyte count. They are slightly smaller than neutrophils and eosinophils at 8–10 µm in diameter. The granules of basophils stain best with basic (alkaline) stains.
In general, basophils intensify the inflammatory response. The granules of basophils release histamines, which contribute to inflammation, and heparin, which opposes blood clotting. High counts of basophils are associated with allergies, parasitic infections, and hypothyroidism. Low counts are associated with pregnancy, stress, and hyperthyroidism.
Agranular Leukocytes
Agranular leukocytes contain smaller, less-visible granules in their cytoplasm than do granular leukocytes. The nucleus is simple in shape, sometimes with an indentation but without distinct lobes. There are two major types of agranulocytes: lymphocytes and monocytes.
Lymphocytes
Lymphocytes are the only formed element of blood that arises from lymphoid stem cells. Although they form initially in the bone marrow, much of their subsequent development and reproduction occurs in the lymphatic tissues. Lymphocytes are the second most common type of leukocyte, accounting for about 20–30 percent of all leukocytes, and are essential for the immune response. The size range of lymphocytes is quite extensive, with some authorities recognizing two size classes and others three. Typically, the large cells are 10–14 µm and have a smaller nucleus-to-cytoplasm ratio and more granules. The smaller cells are typically 6–9 µm with a larger volume of nucleus to cytoplasm, creating a “halo” effect. A few cells may fall outside these ranges, at 14–17 µm. This finding has led to the three size range classification.
The three major groups of lymphocytes include natural killer cells, B cells, and T cells. Natural killer (NK) cells can recognize cells that do not express “self” proteins on their plasma membrane or contain foreign or abnormal markers. These “nonself” cells include cancer cells, cells infected with a virus, and other cells with atypical surface proteins. Thus, they provide generalized, nonspecific immunity. The larger lymphocytes are typically NK cells.
B cells and T cells also called B lymphocytes and T lymphocytes, play prominent roles in defending the body against specific pathogens (disease-causing microorganisms) and are involved in specific immunity. One form of B cells (plasma cells) produces the antibodies or immunoglobulins that bind to specific foreign or abnormal components of plasma membranes. This is also referred to as humoral (body fluid) immunity. T cells provide cellular-level immunity by physically attacking foreign or diseased cells. A memory cell is a variety of both B and T cells that forms after exposure to a pathogen and mounts rapid responses upon subsequent exposures. Unlike other leukocytes, memory cells live for many years. B cells undergo a maturation process in the bone marrow, whereas T cells undergo maturation in the thymus. The site of the maturation process gives rise to the name B and T cells. The functions of lymphocytes are complex and will be covered in detail in the chapter covering the lymphatic system and immunity. Smaller lymphocytes are either B or T cells, although they cannot be differentiated in a normal blood smear.
Abnormally high lymphocyte counts are characteristic of viral infections as well as some types of cancer. Abnormally low lymphocyte counts are characteristic of prolonged (chronic) illness or immunosuppression, including that caused by HIV infection and drug therapies that often involve steroids.
Monocytes
Monocytes originate from myeloid stem cells. They normally represent 2–8 percent of the total leukocyte count. They are typically easily recognized by their large size of 12–20 µm and indented or horseshoe-shaped nuclei. Macrophages are monocytes that have left the circulation and phagocytize debris, foreign pathogens, worn-out erythrocytes, and many other dead, worn out, or damaged cells. Macrophages also release antimicrobial defensins and chemotactic chemicals that attract other leukocytes to the site of an infection. Some macrophages occupy fixed locations, whereas others wander through the tissue fluid.
Abnormally high counts of monocytes are associated with viral or fungal infections, tuberculosis, and some forms of leukemia and other chronic diseases. Abnormally low counts are typically caused by suppression of the bone marrow.
Lifecycle of Leukocytes
Most leukocytes have a relatively short lifespan, typically measured in hours or days. Production of all leukocytes begins in the bone marrow under the influence of CSFs and interleukins. Secondary production and maturation of lymphocytes occurs in specific regions of lymphatic tissue known as germinal centers. Lymphocytes are fully capable of mitosis and may produce clones of cells with identical properties. This capacity enables an individual to maintain immunity throughout life to many threats that have been encountered in the past.
Disorders of Leukocytes – FYI (For Your Information -> you will not be assessed on this content)
- Leukopenia is a condition in which too few leukocytes are produced. If this condition is pronounced, the individual may be unable to ward off disease.
- Excessive leukocyte proliferation is known as leukocytosis. Although leukocyte counts are high, the cells themselves are often nonfunctional, leaving the individual at increased risk for disease.
- Leukemia is cancer involving an abundance of leukocytes. It may involve only one specific type of leukocyte from either the myeloid line (myelocytic leukemia) or the lymphoid line (lymphocytic leukemia). In chronic leukemia, mature leukocytes accumulate and fail to die. In acute leukemia, there is an overproduction of young, immature leukocytes. In both conditions, the cells do not function properly.
- Lymphoma is a form of cancer in which masses of malignant T and/or B lymphocytes collect in lymph nodes, the spleen, the liver, and other tissues. As in leukemia, the malignant leukocytes do not function properly, and the patient is vulnerable to infection. Some forms of lymphoma tend to progress slowly and respond well to treatment. Others tend to progress quickly and require aggressive treatment, without which they are rapidly fatal.
Platelets
You may occasionally see platelets referred to as thrombocytes, but because this name suggests they are a type of cell, it is not accurate. A platelet is not a cell but rather a fragment of the cytoplasm of a cell called a megakaryocyte that is surrounded by a plasma membrane. Megakaryocytes are descended from myeloid stem cells and are large, typically 50–100 µm in diameter, and contain an enlarged, lobed nucleus. Each megakaryocyte releases 2000–3000 platelets during its lifespan. Following platelet release, megakaryocyte remnants, which are little more than a cell nucleus, are consumed by macrophages.
Platelets are relatively small, 2–4 µm in diameter, but numerous, with typically 150,000–160,000 per µL of blood. After entering the circulation, approximately one-third migrate to the spleen for storage for later release in response to any rupture in a blood vessel. They then become activated to perform their primary function, which is to limit blood loss. Platelets remain only about 10 days, then are phagocytized by macrophages.
Platelets are critical to hemostasis, the stoppage of blood flow following damage to a vessel. They also secrete various growth factors essential for the growth and repair of tissue, particularly connective tissue. Infusions of concentrated platelets are now being used in some therapies to stimulate healing.
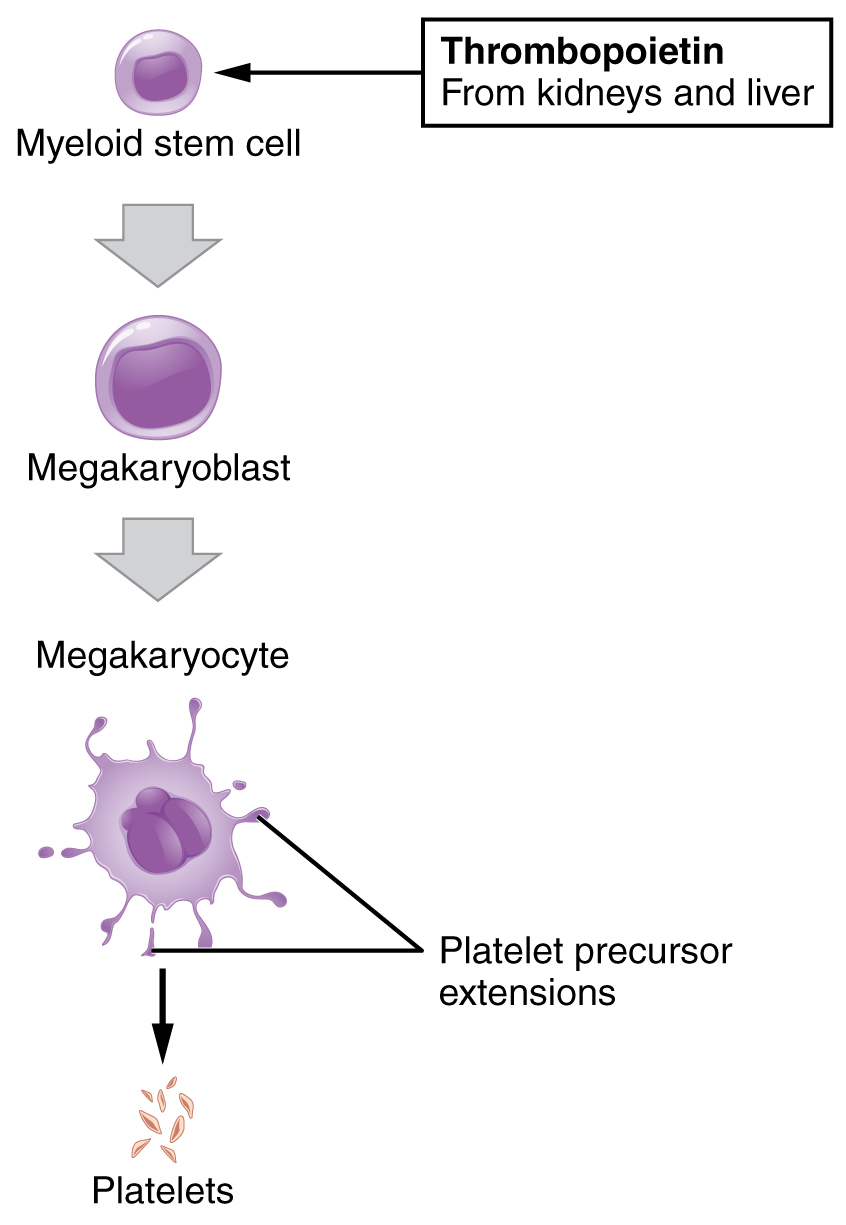
Disorders of Platelets – FYI (For Your Information -> you will not be assessed on this content)
Thrombocytosis is a condition in which there are too many platelets. This may trigger the formation of unwanted blood clots (thrombosis), a potentially fatal disorder. If there is an insufficient number of platelets, called thrombocytopenia, blood may not clot properly, and excessive bleeding may result.
Blood Vessels
Blood is carried through the body via blood vessels. An artery is a blood vessel that carries blood away from the heart, where it branches into ever-smaller vessels. Eventually, the smallest arteries, vessels called arterioles, further branch into tiny capillaries, where nutrients and wastes are exchanged, and then combine with other vessels that exit capillaries to form venules, small blood vessels that carry blood to a vein, a larger blood vessel that returns blood to the heart.
Arteries and veins transport blood in two distinct circuits: the systemic circuit and the pulmonary circuit. Systemic arteries provide blood rich in oxygen to the body’s tissues. The blood returned to the heart through systemic veins has less oxygen since much of the oxygen carried by the arteries has been delivered to the cells. In contrast, in the pulmonary circuit, arteries carry blood low in oxygen exclusively to the lungs for gas exchange. Pulmonary veins then return freshly oxygenated blood from the lungs to the heart to be pumped back into systemic circulation. Although arteries and veins differ structurally and functionally, they share certain features.
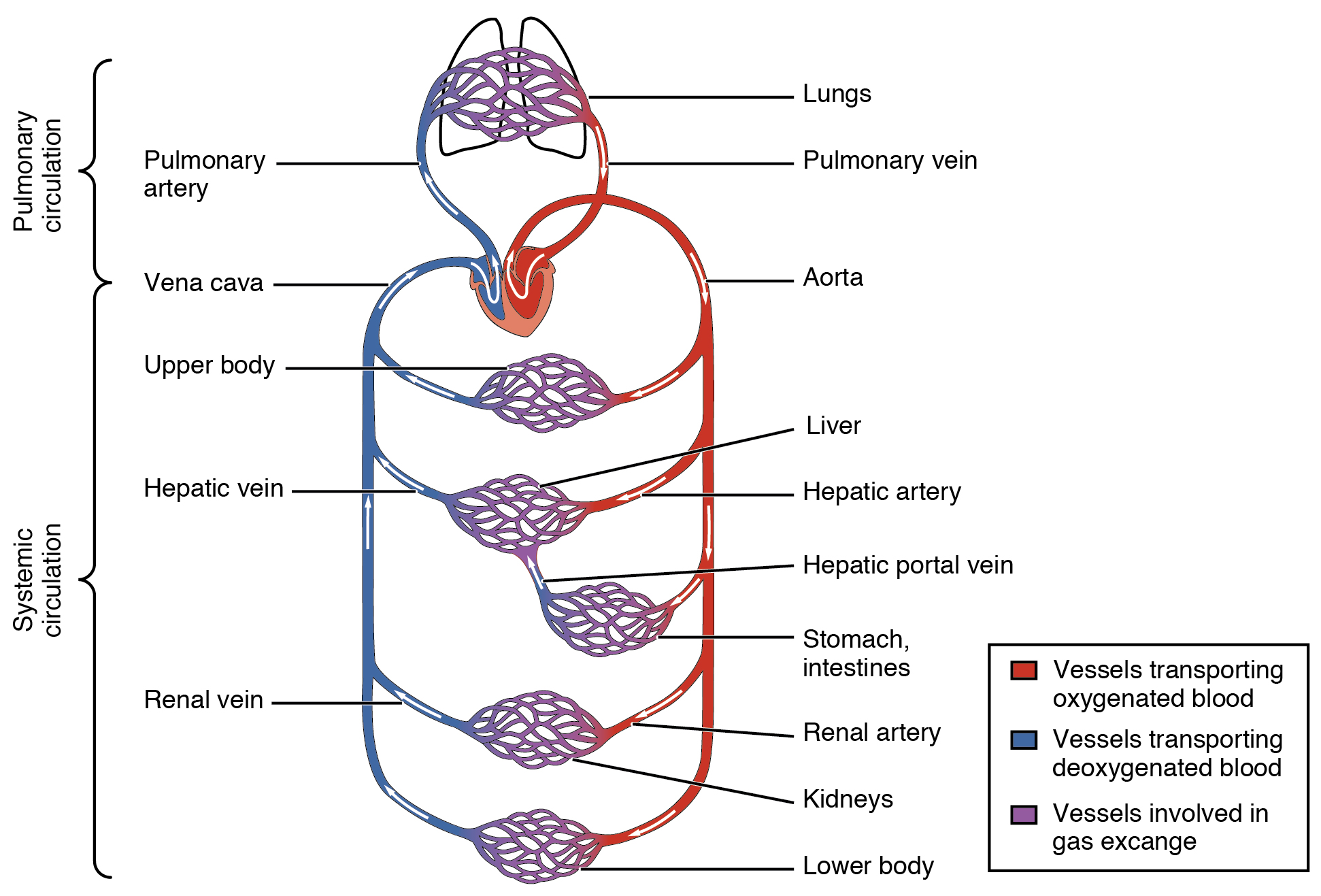
Shared Structures
Different types of blood vessels vary slightly in their structures, but they share the same general features. Arteries and arterioles have thicker walls than veins and venules because they are closer to the heart and receive blood surging at a far greater pressure. Each type of vessel has a lumen—a hollow passageway through which blood flows. Arteries have smaller lumens than veins, a characteristic that helps to maintain the pressure of blood moving through the system. Together, their thicker walls and smaller diameters give arterial lumens a more rounded appearance in cross-section than the lumens of veins.
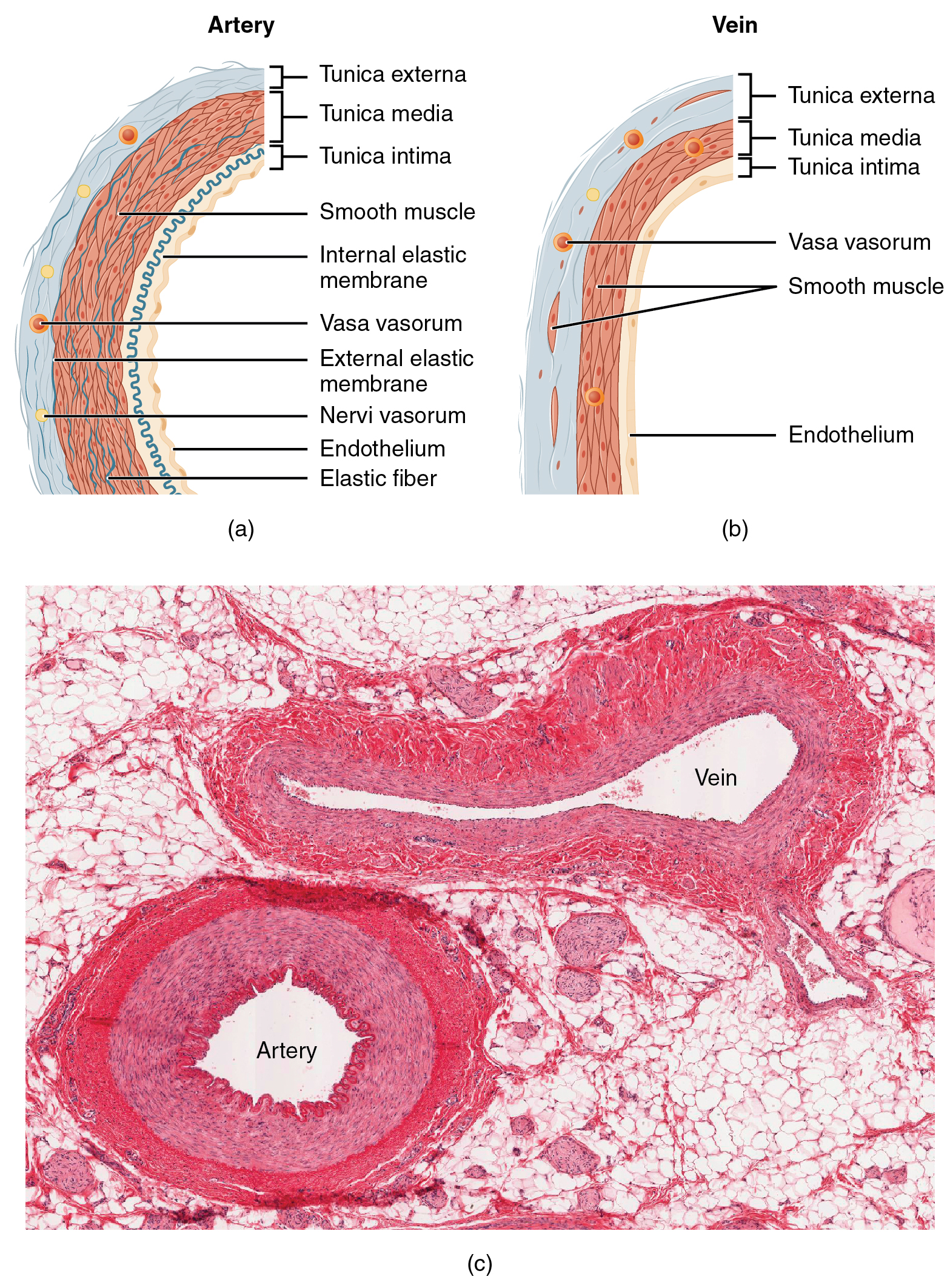
By the time blood has passed through capillaries and entered venules, the pressure initially exerted upon it by heart contractions has diminished. In other words, in comparison to arteries, venules and veins withstand a much lower pressure from the blood that flows through them. Their walls are considerably thinner, and their lumens are correspondingly larger in diameter, allowing more blood to flow with less vessel resistance. In addition, many veins of the body, particularly those of the limbs, contain valves that assist the unidirectional flow of blood toward the heart. This is critical because blood flow becomes sluggish in the extremities due to the lower pressure and the effects of gravity.
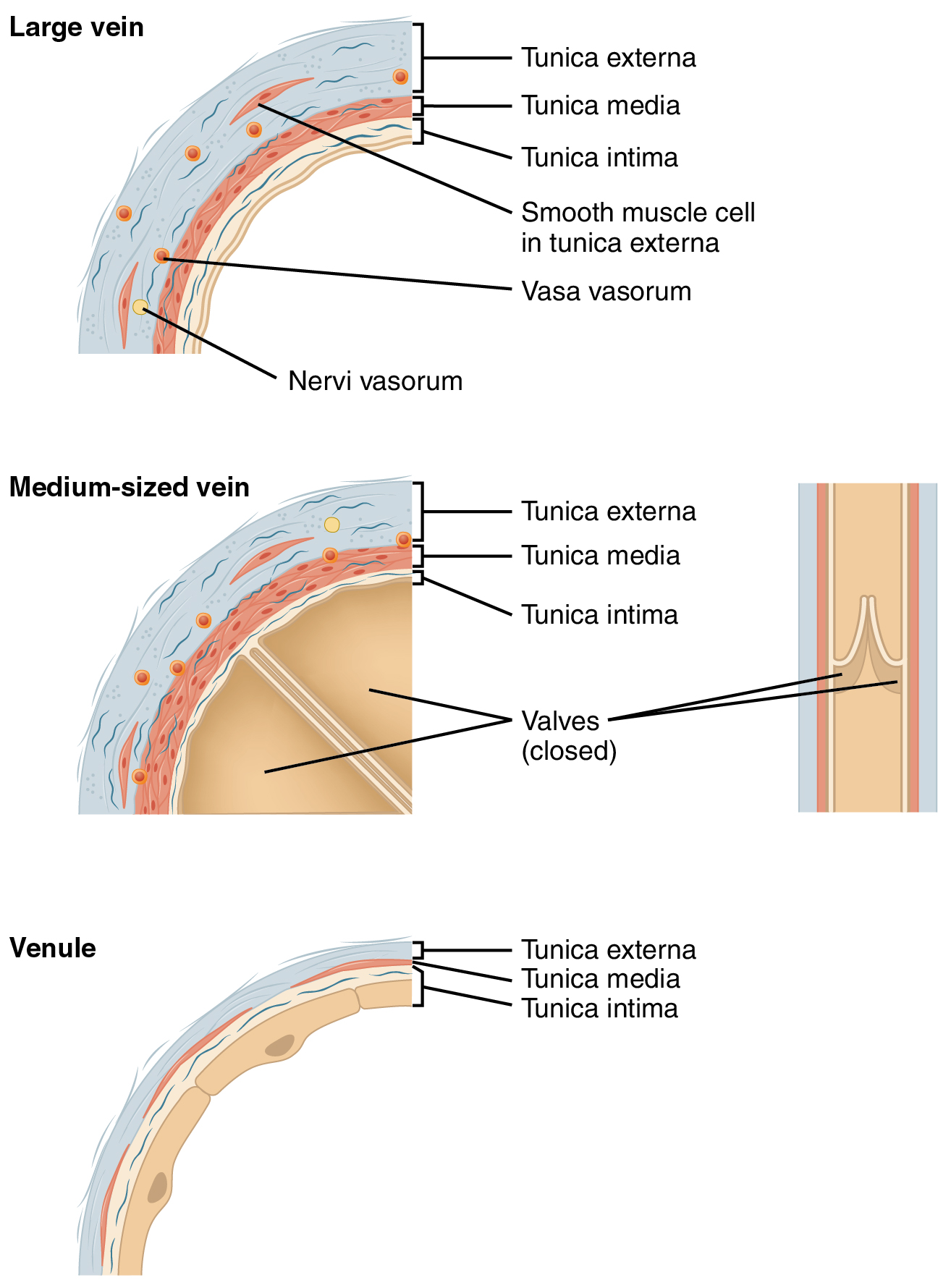
The walls of arteries and veins are largely composed of living cells and their products (including collagenous and elastic fibers); the cells require nourishment and produce waste. Since blood passes through the larger vessels relatively quickly, there is limited opportunity for blood in the lumen of the vessel to provide nourishment to or remove waste from the vessel’s cells. Further, the walls of the larger vessels are too thick for nutrients to diffuse through to all of the cells. Larger arteries and veins contain small blood vessels within their walls known as the vasa vasorum—literally “vessels of the vessel”—to provide them with this critical exchange. Since the pressure within arteries is relatively high, the vasa vasorum must function in the outer layers of the vessel, or the pressure exerted by the blood passing through the vessel would collapse it, preventing any exchange from occurring. The lower pressure within veins allows the vasa vasorum to be located closer to the lumen. The restriction of the vasa vasorum to the outer layers of arteries is thought to be one reason that arterial diseases are more common than venous diseases since its location makes it more difficult to nourish the cells of the arteries and remove waste products. There are also minute nerves within the walls of both types of vessels that control the contraction and dilation of smooth muscle. These minute nerves are known as the nervi vasorum.
Both arteries and veins have the same three distinct tissue layers, called tunics (from the Latin term tunica), for the garments first worn by ancient Romans; the term tunic is also used for some modern garments. From the most interior layer to the outer, these tunics are the tunica intima, the tunica media, and the tunica externa (adventitia).
| Comparison of Tunics in Arteries and Veins | ||
|---|---|---|
| Arteries | Veins | |
| General appearance | Thick walls with small lumens Generally appear rounded |
Thin walls with large lumens Generally appear flattened |
| Tunica intima | Endothelium usually appears wavy due to constriction of smooth muscle Internal elastic membrane present in larger vessels |
Endothelium appears smooth Internal elastic membrane absent |
| Tunica media | Normally the thickest layer in arteries Smooth muscle cells and elastic fibers predominate (the proportions of these vary with distance from the heart) External elastic membrane present in larger vessels |
Normally thinner than the tunica externa Smooth muscle cells and collagenous fibers predominate Nervi vasorum and vasa vasorum present External elastic membrane absent |
| Tunica externa | Normally thinner than the tunica media in all but the largest arteries Collagenous and elastic fibers Nervi vasorum and vasa vasorum present |
Normally the thickest layer in veins Collagenous and smooth fibers predominate Some smooth muscle fibers Nervi vasorum and vasa vasorum present |
Tunica Intima
The tunica intima (also called the tunica interna) is composed of epithelial and connective tissue layers. Lining the tunica intima is the specialized simple squamous epithelium called the endothelium, which is continuous throughout the entire vascular system, including the lining of the chambers of the heart. Damage to this endothelial lining and exposure of blood to the collagenous fibers beneath is one of the primary causes of clot formation.
Next to the endothelium is the basement membrane or basal lamina, which effectively binds the endothelium to the connective tissue. The basement membrane provides strength while maintaining flexibility, and it is permeable, allowing materials to pass through it. The thin outer layer of the tunica intima contains a small amount of areolar connective tissue that consists primarily of elastic fibers to provide the vessel with additional flexibility; it also contains some collagenous fibers to provide additional strength.
In larger arteries, there is also a thick, distinct layer of elastic fibers known as the internal elastic membrane (also called the internal elastic lamina) at the boundary with the tunica media. Like the other components of the tunica intima, the internal elastic membrane provides structure while allowing the vessel to stretch. It is permeated with small openings that allow the exchange of materials between the tunics. The internal elastic membrane is not apparent in veins. In addition, many veins, particularly in the lower limbs, contain valves formed by sections of thickened endothelium reinforced with connective tissue, extending into the lumen.
Under the microscope, the lumen and the entire tunica intima of a vein will appear smooth. In contrast, those of an artery will normally appear wavy because of the partial constriction of the smooth muscle in the tunica media, the next layer of blood vessel walls.
Tunica Media
The tunica media is the substantial middle layer of the vessel wall. It is generally the thickest layer in arteries, and it is much thicker in arteries than it is in veins. The tunica media consists of layers of smooth muscle supported by connective tissue primarily made up of elastic fibers, most of which are arranged in circular sheets. Toward the outer portion of the tunic, there are also layers of longitudinal muscle. Contraction and relaxation of the circular muscles decrease and increase the diameter of the vessel lumen, respectively. Specifically, in arteries, vasoconstriction decreases blood flow as the smooth muscle in the walls of the tunica media contracts, making the lumen narrower and increasing blood pressure. Similarly, vasodilation increases blood flow as the smooth muscle relaxes, allowing the lumen to widen and blood pressure to drop.
The smooth muscle layers of the tunica media are supported by a framework of collagenous fibers that also binds the tunica media to the inner and outer tunics. Along with the collagenous fibers are large numbers of elastic fibers that appear as wavy lines in prepared slides. Separating the tunica media from the outer tunica externa in larger arteries is the external elastic membrane (also called the external elastic lamina), which appears wavy in slides. This structure is not usually seen in smaller arteries, nor is it seen in veins.
Tunica Externa
The outer tunic, the tunica externa (also called the tunica adventitia), is a substantial sheath of connective tissue composed primarily of collagenous fibers. Some bands of elastic fibers are found here as well. The tunica externa in veins also contains groups of smooth muscle fibers. This is normally the thickest tunic in veins and may be thicker than the tunica media in some larger arteries. The outer layers of the tunica externa are not distinct but rather blend with the surrounding connective tissue outside the vessel, helping to hold the vessel in relative position. If you can palpate some of the superficial veins on your upper limbs and try to move them, you will find that the tunica externa prevents this. If the tunica externa did not hold the vessel in place, any movement would likely disrupt blood flow.
Arteries
An artery is a blood vessel that conducts blood away from the heart. All arteries have relatively thick walls that can withstand the high pressure of blood ejected from the heart. However, those close to the heart have the thickest walls, containing a high percentage of elastic fibers in all three of their tunics. This type of artery is known as an elastic artery. Vessels larger than 10 mm in diameter are typically elastic. Their abundant elastic fibers allow them to expand as blood pumped from the ventricles passes through them and then to recoil after the surge has passed. If artery walls were rigid and unable to expand and recoil, their resistance to blood flow would greatly increase. Blood pressure would rise to even higher levels, which would, in turn, require the heart to pump harder to increase the volume of blood expelled by each pump (the stroke volume) and maintain adequate pressure and flow. Artery walls would have to become even thicker in response to this increased pressure. The elastic recoil of the vascular wall helps to maintain the pressure gradient that drives the blood through the arterial system. An elastic artery is also known as a conducting artery because the large diameter of the lumen enables it to accept a large volume of blood from the heart and conduct it to smaller branches.
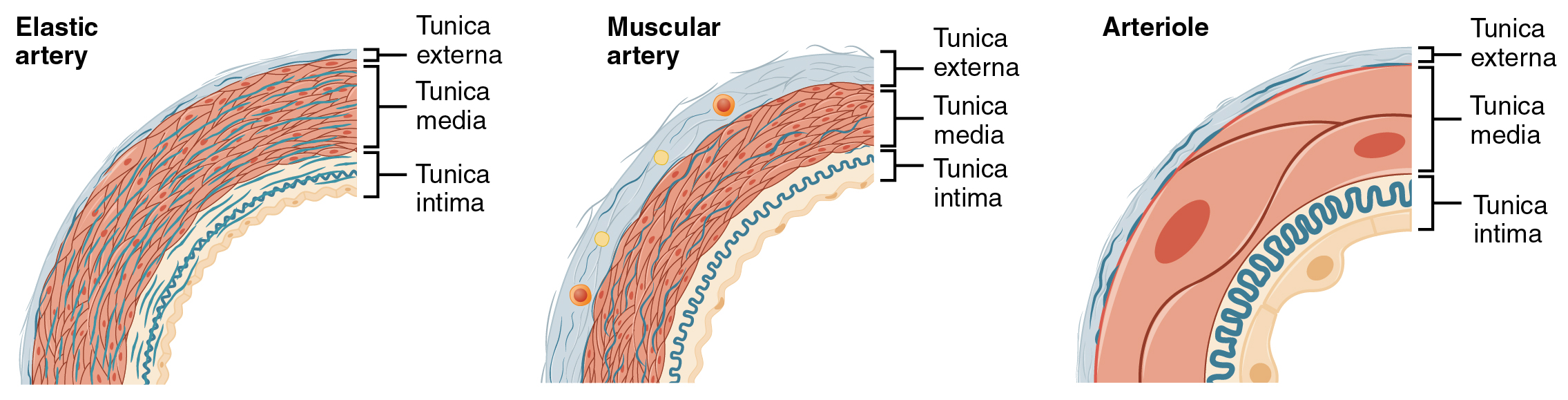
Farther from the heart, where the surge of blood has dampened, the percentage of elastic fibers in an artery’s tunica intima decreases, and the amount of smooth muscle in its tunica media increases. The artery at this point is described as a muscular artery. The diameter of muscular arteries typically ranges from 0.1 mm to 10 mm. Their thick tunica media allows muscular arteries to play a leading role in vasoconstriction. In contrast, their decreased quantity of elastic fibers limits their ability to expand. Fortunately, because the blood pressure has eased by the time it reaches these more distant vessels, elasticity has become less important.
Notice that although the distinctions between elastic and muscular arteries are important, there is no “line of demarcation” where an elastic artery suddenly becomes muscular. Rather, there is a gradual transition as the vascular tree repeatedly branches. In turn, muscular arteries branch to distribute blood to the vast network of arterioles. For this reason, a muscular artery is also known as a distributing artery.
Arterioles
An arteriole is a very small artery that leads to a capillary. Arterioles have the same three tunics as the larger vessels, but the thickness of each is greatly diminished. The critical endothelial lining of the tunica intima is intact. The tunica media is restricted to one or two smooth muscle cell layers in thickness. The tunica externa remains but is very thin.
With a lumen averaging 30 micrometers or less in diameter, arterioles are critical in slowing down—or resisting—blood flow and, thus, causing a substantial drop in blood pressure. The muscle fibers in arterioles are normally slightly contracted, causing arterioles to maintain a consistent muscle tone—in this case, referred to as vascular tone—in a similar manner to the muscular tone of skeletal muscle. In reality, all blood vessels exhibit vascular tone due to the partial contraction of smooth muscle. The importance of the arterioles is that they will be the primary site of both resistance and regulation of blood pressure. The precise diameter of the lumen of an arteriole at any given moment is determined by neural and chemical controls, and vasoconstriction and vasodilation in the arterioles are the primary mechanisms for the distribution of blood flow.
Capillaries
A capillary is a microscopic channel that supplies blood to the tissues themselves, a process called perfusion. The exchange of gases and other substances occurs in the capillaries between the blood and the surrounding cells and their tissue fluid (interstitial fluid). A capillary lumen diameter ranges from 5–10 micrometers; the smallest are just barely wide enough for an erythrocyte to squeeze through.
The wall of a capillary consists of the endothelial layer surrounded by a basement membrane with occasional smooth muscle fibers. There is some variation in wall structure: In a large capillary, several endothelial cells bordering each other may line the lumen; in a small capillary, there may be only a single cell layer that wraps around to contact itself.
For capillaries to function, their walls must be leaky, allowing substances to pass through. There are three major types of capillaries, which differ according to their degree of “leakiness:” continuous, fenestrated, and sinusoid capillaries.
Continuous Capillaries
The most common type of capillary, the continuous capillary, is found in almost all vascularized tissues. Continuous capillaries are characterized by a complete endothelial lining with tight junctions between endothelial cells. Although a tight junction is usually impermeable and only allows for the passage of water and ions, they are often incomplete in capillaries, leaving intercellular clefts that allow for the exchange of water and other very small molecules between the blood plasma and the interstitial fluid. Substances that can pass between cells include metabolic products, such as glucose, water, and small hydrophobic molecules like gases and hormones, and various leukocytes.
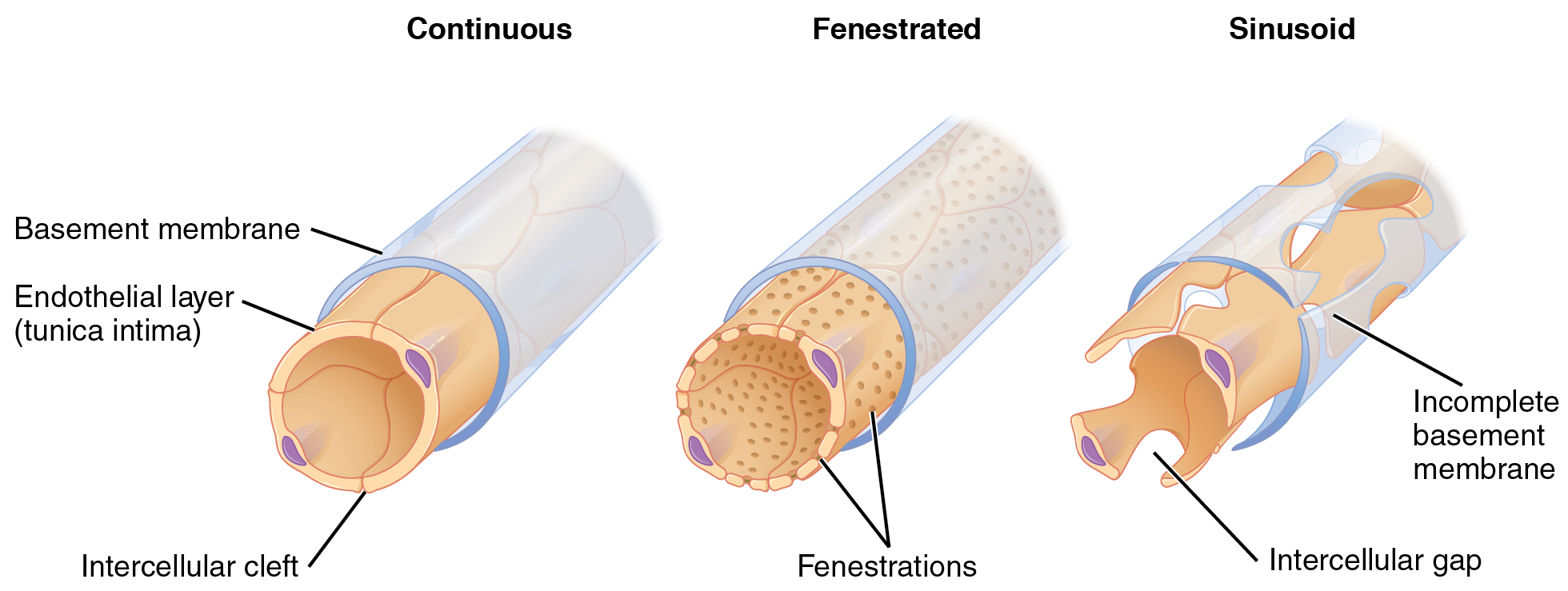
Fenestrated Capillaries
A fenestrated capillary is one that has pores (or fenestrations) in addition to tight junctions in the endothelial lining. These make the capillary permeable to larger molecules. The number of fenestrations and their degree of permeability vary, however, according to their location. Fenestrated capillaries are common in the small intestine, the primary site of nutrient absorption, and the kidneys, which filter the blood. They are also found in the choroid plexus of the brain and many endocrine structures, including the hypothalamus, pituitary, pineal, and thyroid glands.
Sinusoid Capillaries
A sinusoid capillary (or sinusoid) is the least common type of capillary. Sinusoid capillaries are flattened, and they have extensive intercellular gaps and incomplete basement membranes, in addition to intercellular clefts and fenestrations. This gives them an appearance not unlike Swiss cheese. These very large openings allow for the passage of the largest molecules, including plasma proteins and even cells. Blood flow through sinusoids is very slow, allowing more time for the exchange of gases, nutrients, and wastes. Sinusoids are found in the liver and spleen, bone marrow, lymph nodes (where they carry lymph, not blood), and many endocrine glands, including the pituitary and adrenal glands. Without these specialized capillaries, these organs would not be able to provide their myriad of functions.
Metarterioles and Capillary Beds
A metarteriole is a type of vessel that has structural characteristics of both an arteriole and a capillary. Slightly larger than the typical capillary, the smooth muscle of the tunica media of the metarteriole is not continuous but forms rings of smooth muscle (sphincters) before the entrance to the capillaries. Each metarteriole arises from a terminal arteriole and branches to supply blood to a capillary bed that may consist of 10–100 capillaries.
The precapillary sphincters, circular smooth muscle cells that surround the capillary at its origin with the metarteriole, tightly regulate blood flow from a metarteriole to the capillaries it supplies. Their function is critical: If all of the capillary beds in the body were to open simultaneously, they would collectively hold every drop of blood in the body, and there would be none in the arteries, arterioles, venules, veins, or the heart itself. Normally, the precapillary sphincters are closed. When the surrounding tissues need oxygen and have excess waste products, the precapillary sphincters open, allowing blood to flow through and exchange to occur before closing once more. If all of the precapillary sphincters in a capillary bed are closed, blood will flow from the metarteriole directly into a thoroughfare channel and then into the venous circulation, bypassing the capillary bed entirely. This creates what is known as a vascular shunt. In addition, an arteriovenous anastomosis may bypass the capillary bed and lead directly to the venous system.
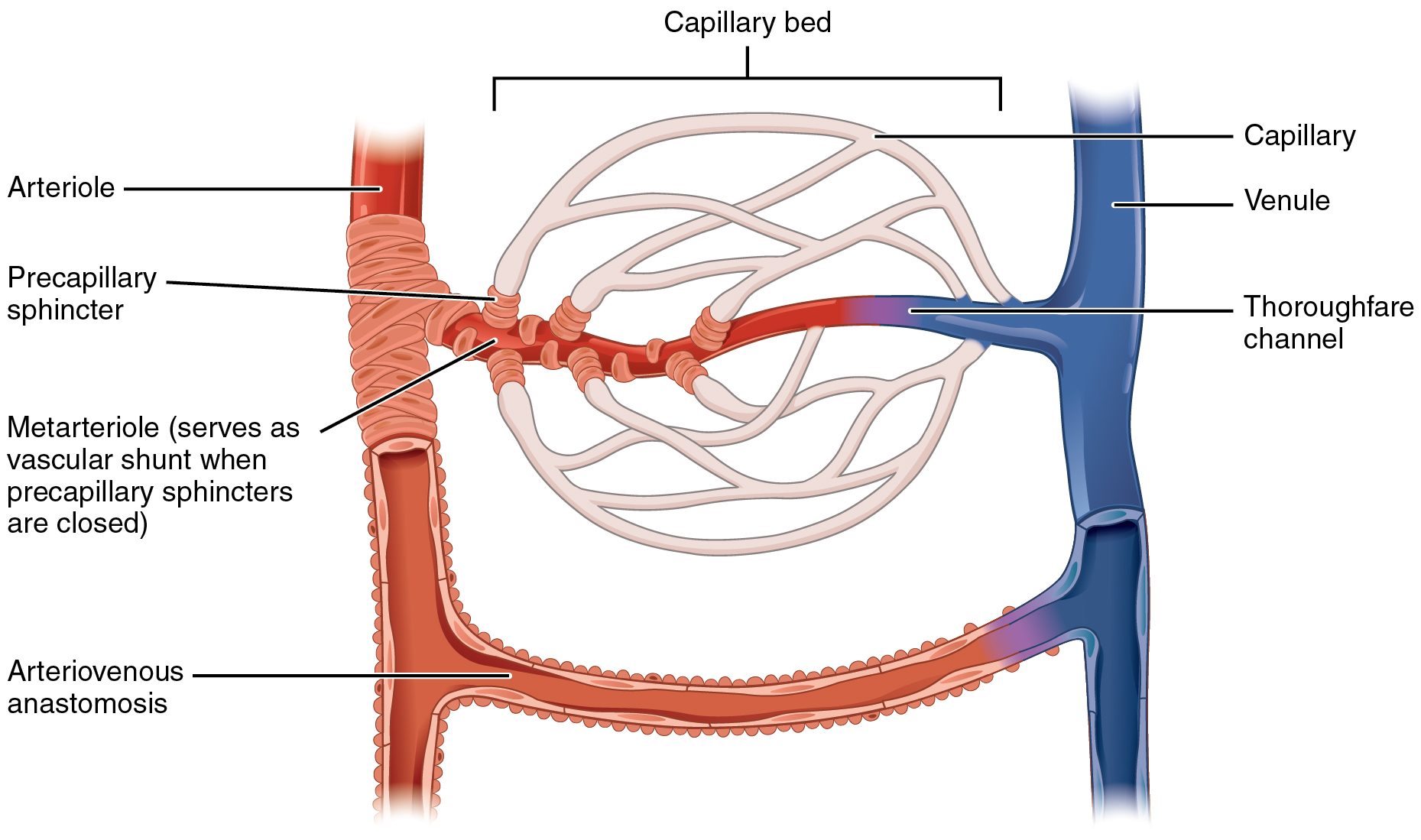
Venules
A venule is an extremely small vein, generally 8–100 micrometers in diameter. Postcapillary venules join multiple capillaries exiting from a capillary bed. Multiple venules join to form veins. The walls of venules consist of endothelium, a thin middle layer with a few muscle cells and elastic fibers, plus an outer layer of connective tissue fibers that constitute a very thin tunica externa. Venules and capillaries are the primary sites of emigration or diapedesis. The white blood cells adhere to the endothelial lining of the vessels and then squeeze through adjacent cells to enter the tissue fluid.
Veins
A vein is a blood vessel that conducts blood toward the heart. Compared to arteries, veins are thin-walled vessels with large and irregular lumens. Because they are low-pressure vessels, larger veins are commonly equipped with valves that promote the unidirectional flow of blood toward the heart and prevent backflow toward the capillaries caused by the inherent low blood pressure in veins as well as the pull of gravity.
Comparison of Veins and Venules
| Comparison of Arteries and Veins | ||
|---|---|---|
| Arteries | Veins | |
| Direction of blood flow | Conducts blood away from the heart | Conducts blood toward the heart |
| General appearance | Rounded | Irregular, often collapsed |
| Pressure | High | Low |
| Wall thickness | Thick | Thin |
| Relative oxygen concentration | Higher in systemic arteries Lower in pulmonary arteries |
Lower in systemic veins Higher in pulmonary veins |
| Valves | Not present | Present most commonly in limbs and veins inferior to the heart |
Disorders of the Cardiovascular System: Edema and Varicose Veins
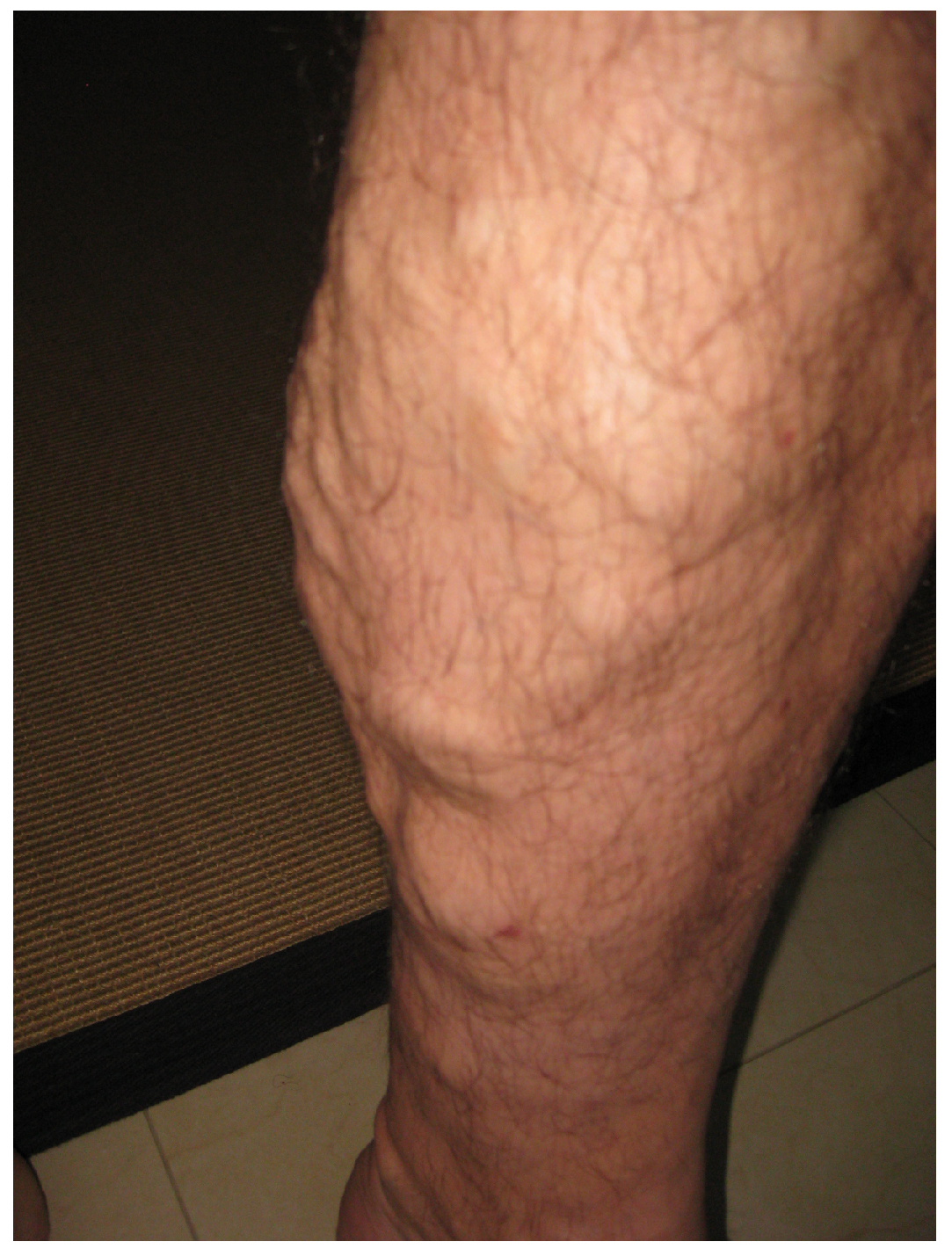
Edema may be accompanied by varicose veins, especially in the superficial veins of the legs. This disorder arises when defective valves allow blood to accumulate within the veins, causing them to distend, twist, and become visible on the surface of the integument. Varicose veins may occur in both sexes but are more common in women and are often related to pregnancy. More than simple cosmetic blemishes, varicose veins are often painful and sometimes itchy or throbbing. Without treatment, they tend to grow worse over time. The use of a support hose and elevating the feet and legs whenever possible may help alleviate this condition. Laser surgery and interventional radiologic procedures can reduce the size and severity of varicose veins. Severe cases may require conventional surgery to remove the damaged vessels. As there are typically redundant circulation patterns, anastomoses, for the smaller and more superficial veins, removal does not typically impair the circulation. There is evidence that patients with varicose veins suffer a greater risk of developing a thrombus or clot.
Fun Fact – Veins as Blood Reservoirs (-> you will not be assessed on this content)
In addition to their primary function of returning blood to the heart, veins may be considered blood reservoirs since systemic veins contain approximately 64 percent of the blood volume at any given time. Their ability to hold this much blood is due to their high capacitance, that is, their capacity to distend (expand) readily to store a high volume of blood, even at a low pressure. The large lumens and relatively thin walls of veins make them far more distensible than arteries; thus, they are said to be capacitance vessels.
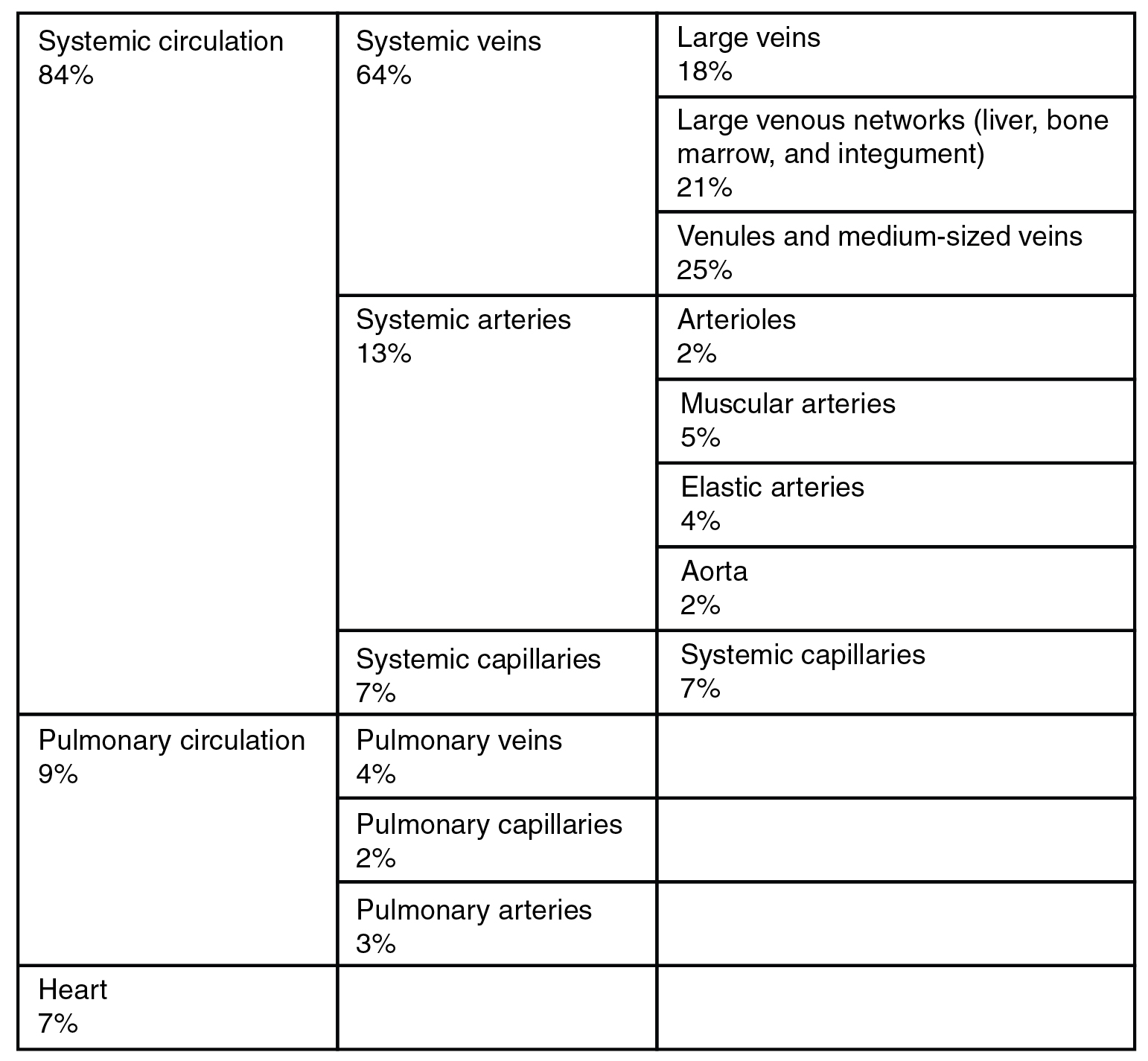
When blood flow needs to be redistributed to other portions of the body, the vasomotor center located in the medulla oblongata sends sympathetic stimulation to the smooth muscles in the walls of the veins, causing constriction—or in this case, venoconstriction. Less dramatic than the vasoconstriction seen in smaller arteries and arterioles, venoconstriction may be likened to a “stiffening” of the vessel wall. This increases pressure on the blood within the veins, speeding its return to the heart. As you will note in the table above, approximately 21 percent of the venous blood is located in venous networks within the liver, bone marrow, and integument. This volume of blood is referred to as venous reserve. Through venoconstriction, this “reserve” volume of blood can get back to the heart more quickly for redistribution to other parts of the circulation.

