Module 5: Cartilage and Bone
Learning Objectives:
By the end of this module, students will be able to:
- Classify the three types of cartilage based on the fibers that comprise them.
- Explain the two methods of cartilage growth.
- Describe the characteristics of the different types of bones.
- Compare and contrast the features of bone and cartilage.
- Summarize the two types of ossification.
- Describe the process of bone remodeling and repair.
- Explain how bone architecture can change with age.
Terms to Know
|
Bone
|
Bone (continued)
Cartilage
|
Two major forms of supportive connective tissue, cartilage, and bone, allow the body to maintain its posture and protect internal organs.
Cartilage
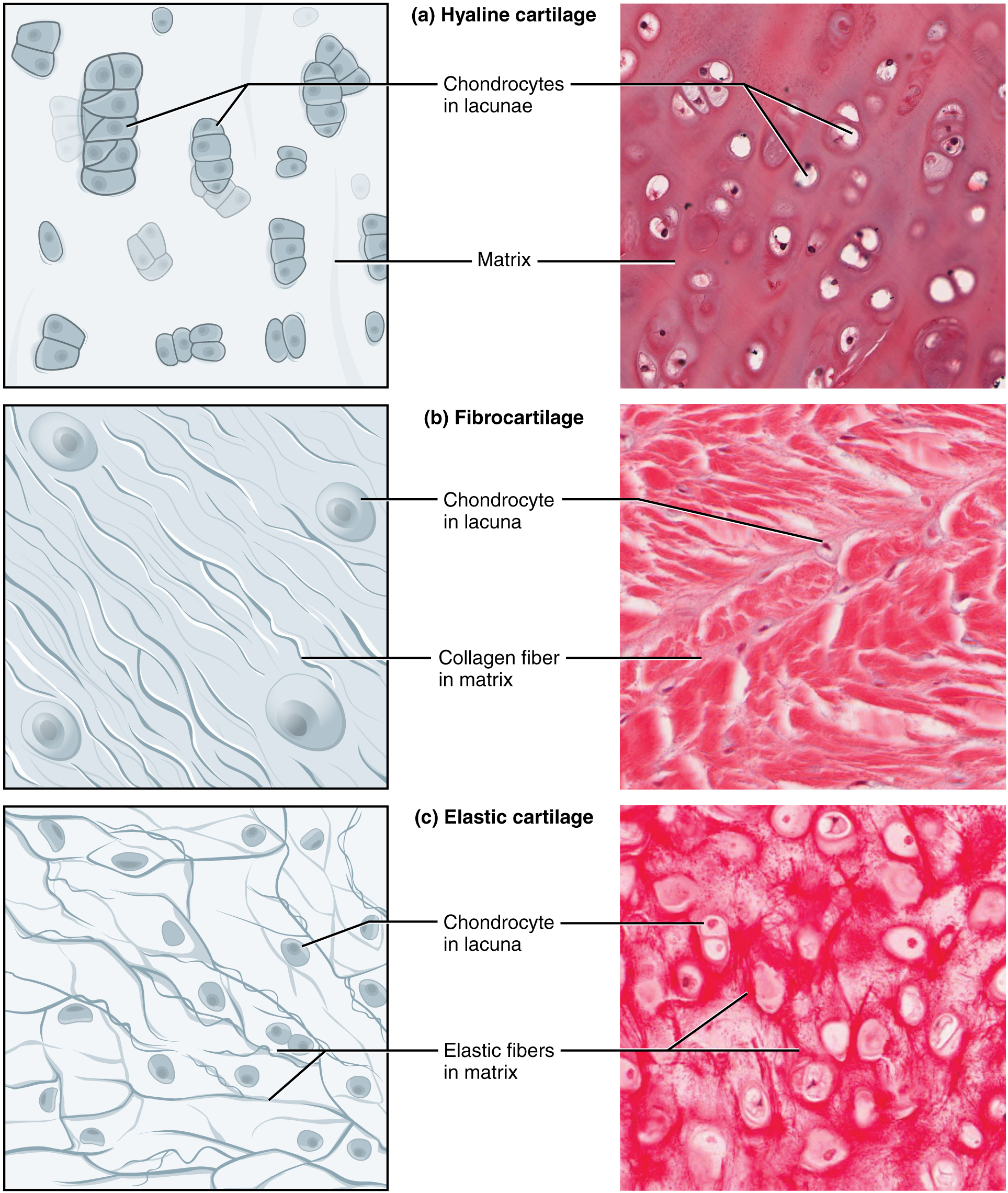
The distinctive appearance of cartilage is due to carbohydrates called chondroitin sulfates. Embedded within the cartilage matrix are chondrocytes or cartilage cells, and the spaces they occupy are called lacunae (singular = lacuna). A layer of dense irregular connective tissue, the perichondrium, encapsulates the cartilage. Cartilaginous tissue is avascular. Thus all nutrients need to diffuse through the matrix to reach the chondrocytes. This factor contributes to the prolonged healing (or lack of healing) of cartilaginous tissues.
The three main types of cartilage tissue are hyaline cartilage, fibrocartilage, and elastic cartilage. Hyaline cartilage, the most common type of cartilage in the body, consists of short and dispersed collagen fibers. Under the microscope, tissue samples appear clear. The surface of hyaline cartilage is smooth. Both strong and flexible, it is found in the rib cage and nose and covers bones where they meet to form moveable joints. It makes up a template of the embryonic skeleton before bone formation. A plate of hyaline cartilage at the ends of bone (growth plate) allows continued growth until adulthood. Fibrocartilage is tough because it has thick bundles of collagen fibers dispersed through its matrix. Menisci in the knee joint and the intervertebral discs are examples of fibrocartilage. Elastic cartilage contains elastic fibers as well as collagen. This tissue gives rigid support as well as elasticity. Tug gently at your ear lobes, and notice that the lobes return to their initial shape. The external ear contains elastic cartilage.
Bone
Bone is the hardest connective tissue. It protects internal organs and supports the body. Bone’s rigid extracellular matrix contains mostly collagen fibers embedded in a mineralized ground substance containing hydroxyapatite, a form of calcium phosphate. Both components of the matrix, organic and inorganic, contribute to the unusual properties of bone. Without collagen, bones would be brittle and shatter easily. Without mineral crystals, bones would flex and provide little support. Osteocytes, bone cells like chondrocytes, are located within lacunae. The histology of transverse tissue from long bone shows a typical arrangement of osteocytes in concentric circles around a central canal. Bone is a highly vascularized tissue. Unlike cartilage, bone tissue can recover from injuries in a relatively short time.
Spongy (cancellous) bone looks like a sponge under the microscope and contains empty spaces between trabeculae, or arches of bone proper. It is lighter than compact bone and is found in the interior of some bones and at the end of long bones. Compact bone is solid and has greater structural strength.
Bone, or osseous tissue, is a hard, dense connective tissue that forms most of the adult skeleton, the body’s support structure. In the areas of the skeleton where bones move (for example, the ribcage and joints), cartilage, a semi-rigid form of connective tissue, provides flexibility and smooth surfaces for movement. The skeletal system is the body system composed of bones and cartilage and performs the following critical functions for the human body:
- supports the body
- facilitates movement
- protects internal organs
- produces blood cells
- stores and releases minerals and fat
Support, Movement, and Protection
The most apparent functions of the skeletal system are the gross functions—those visible by observation. Simply by looking at a person, you can see how the bones support, facilitate movement, and protect the human body.
Just as the steel beams of a building provide a scaffold to support its weight, the bones and cartilage of your skeletal system compose the scaffold that supports the rest of your body. Without the skeletal system, you would be a limp mass of organs, muscle, and skin.
Bones also facilitate movement by serving as points of attachment for your muscles. While some bones only serve as a support for the muscles, others also transmit the forces produced when your muscles contract. From a mechanical point of view, bones act as levers, and joints serve as fulcrums. Unless a muscle spans a joint and contracts, a bone is not going to move.
Bones Support Movement

Mineral Storage, Energy Storage, and Hematopoiesis
On a metabolic level, bone tissue performs several critical functions. For one, the bone matrix acts as a reservoir for several minerals important to the body’s functioning, especially calcium and phosphorus. These minerals, incorporated into bone tissue, can be released back into the bloodstream to maintain levels needed to support physiological processes. For example, calcium ions are essential for muscle contractions and controlling the flow of other ions involved in the transmission of nerve impulses.
Bone also serves as a site for fat storage and blood cell production. The softer connective tissue that fills the interior of most bone is referred to as bone marrow. There are two types of bone marrow: yellow marrow and red marrow. Yellow marrow contains adipose tissue; the triglycerides stored in the adipocytes of the tissue can serve as a source of energy. Red marrow is where hematopoiesis, the production of blood cells, takes place. Red blood cells, white blood cells, and platelets are all produced in the red marrow.
Bone Cells and Tissue
Bone contains a relatively small number of cells entrenched in a matrix of collagen fibers that provide a surface for inorganic salt crystals to adhere to. These salt crystals form when calcium phosphate and calcium carbonate combine to create hydroxyapatite, incorporating other inorganic salts like magnesium hydroxide, fluoride, and sulfate as it crystallizes, or calcifies, on the collagen fibers. The hydroxyapatite crystals give bones their hardness and strength, while the collagen fibers give them flexibility so that they are not brittle.
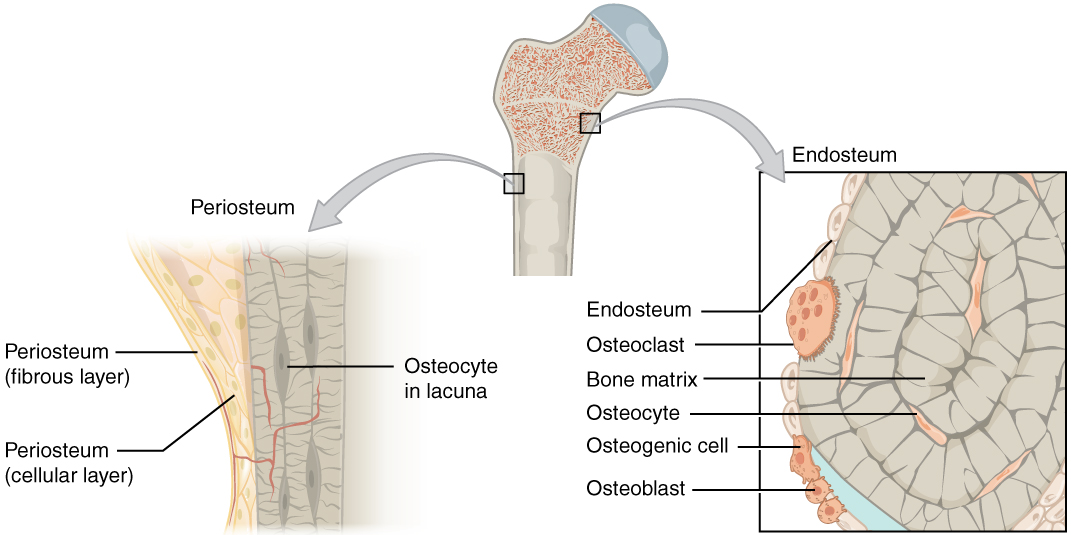
Although bone cells compose a small amount of the bone volume, they are crucial to bones’ function. Four types of cells are found within bone tissue: osteoblasts, osteocytes, osteogenic cells, and osteoclasts.
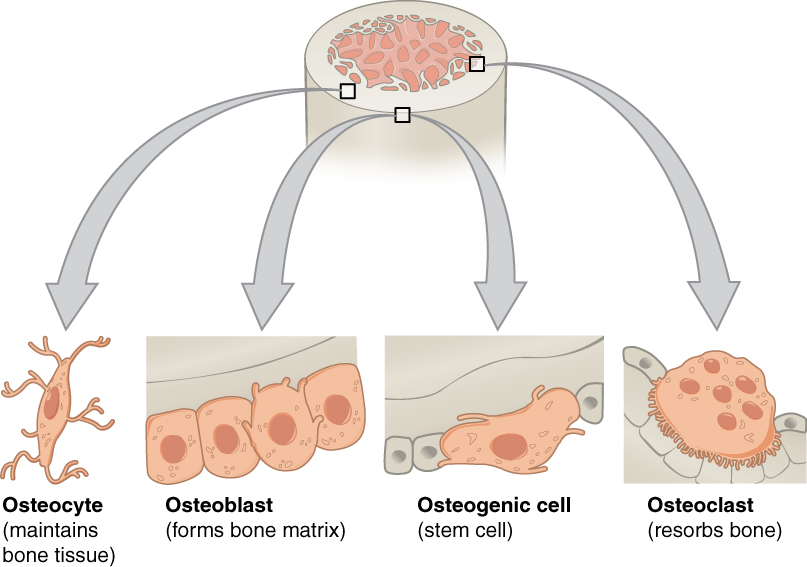
The osteoblast is the bone cell responsible for forming new bone and is found in the growing portions of bone, including the periosteum and endosteum. Osteoblasts, which do not divide, synthesize, and secrete the collagen matrix and calcium salts. As the secreted matrix surrounding the osteoblast calcifies, the osteoblast becomes trapped within it; thus, it changes in structure and becomes an osteocyte, the primary cell of mature bone and the most common type of bone cell. Each osteocyte is located in a space called a lacuna and is surrounded by bone tissue. Osteocytes maintain the mineral concentration of the matrix via the secretion of enzymes. Like osteoblasts, osteocytes lack mitotic activity. They can communicate with each other and receive nutrients via long cytoplasmic processes that extend through canaliculi (singular = canaliculus), channels within the bone matrix.
If osteoblasts and osteocytes are incapable of mitosis, then how are they replenished when old ones die? The answer lies in the properties of a third category of bone cells—the osteogenic cell. These osteogenic cells are undifferentiated with high mitotic activity, and they are the only bone cells that divide. Immature osteogenic cells are found in the deep layers of the periosteum and the marrow. They differentiate and develop into osteoblasts.
The dynamic nature of bone means that new tissue is constantly formed, and old, injured, or unnecessary bone is dissolved for repair or calcium release. The cell responsible for bone resorption, or breakdown, is the osteoclast. They are found on bone surfaces, are multinucleated, and originate from monocytes and macrophages, two types of white blood cells, not from osteogenic cells. Osteoclasts are continually breaking down old bone, while osteoblasts are continually forming new bone. The ongoing balance between osteoblasts and osteoclasts is responsible for the constant but subtle reshaping of bone.
| Bone Cells | ||
|---|---|---|
| Cell type | Function | Location |
| Osteogenic cells | Develop into osteoblasts | Deep layers of the periosteum and the marrow |
| Osteoblasts | Bone formation | Growing portions of bone, including periosteum and endosteum |
| Osteocytes | Maintain mineral concentration of matrix | Entrapped in matrix |
| Osteoclasts | Bone resorption | Bone surfaces and at sites of old, injured, or unneeded bone |
Bone Classification
*The information in the section will be covered in the assignment only*
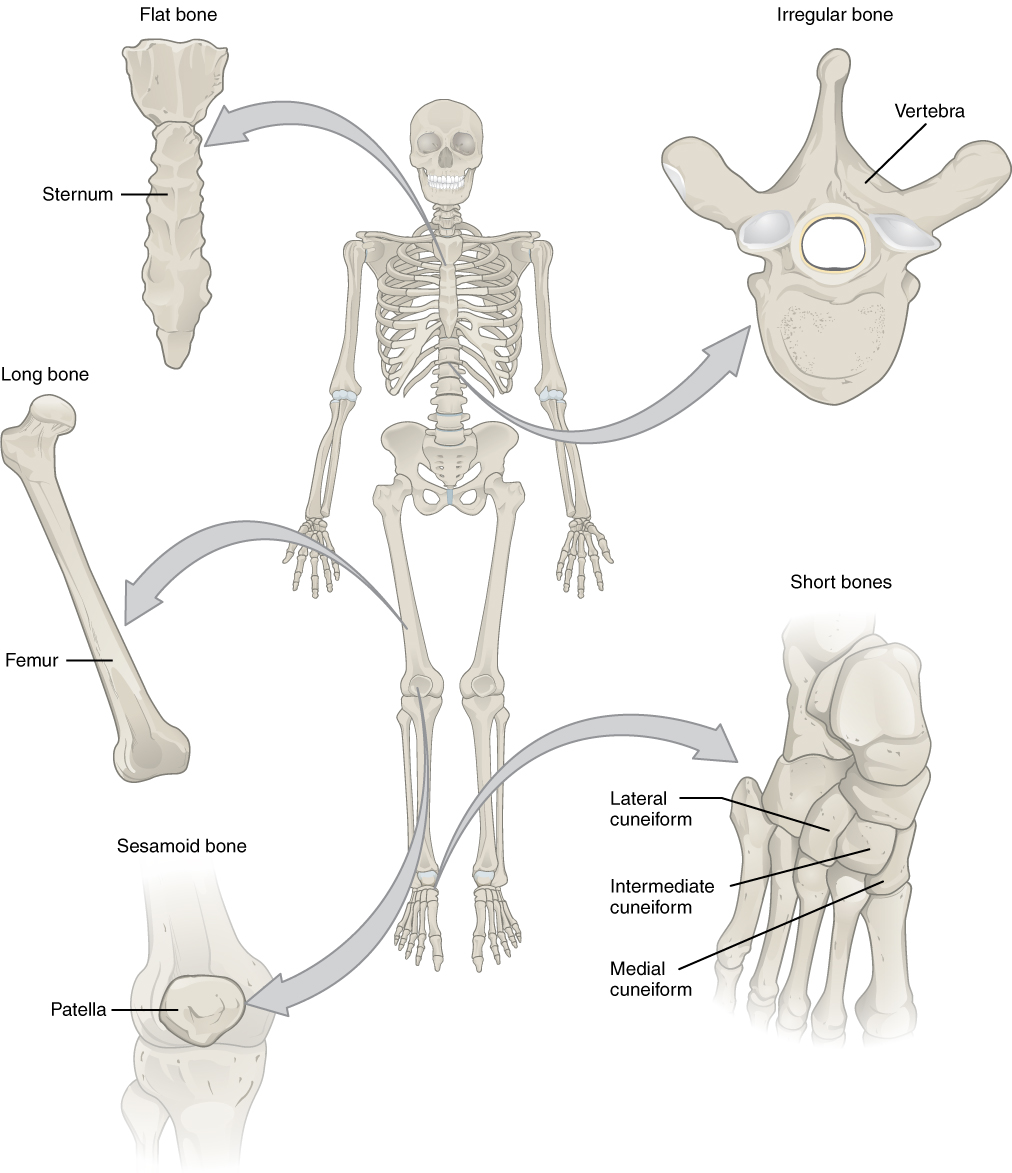
The 206 bones that compose the adult skeleton are divided into categories based on their shapes. Their shapes and their functions are related such that each categorical shape of bone has a distinct function. The image below shows the most common categories of bones **(two are not included in this picture – sutural bones (->>we will not discuss these) and pneumatized bones).
Classifications of Bones
Long Bones
A long bone is one that is cylindrical in shape, being longer than it is wide. However, keep in mind that the term describes the shape of a bone, not its size. Long bones are found in the arms (humerus, ulna, radius) and legs (femur, tibia, fibula), as well as in the fingers (metacarpals, phalanges) and toes (metatarsals, phalanges). Long bones function as levers; they move when muscles contract.
Short Bones
A short bone is cube-like in shape, being approximately equal in length, width, and thickness. The only short bones in the human skeleton are in the carpals of the wrists and the tarsals of the ankles. Short bones provide stability and support as well as some limited motion.
Flat Bones
The term “flat bone” is somewhat of a misnomer because, although a flat bone is typically thin, it is often curved. Examples include the cranial (skull) bones, the scapulae (shoulder blades), the sternum (breastbone), and the ribs. Flat bones serve as points of attachment for muscles and often protect internal organs.
Irregular Bones
An irregular bone does not have any easily characterized shape and, therefore, does not fit any other classification. These bones tend to have more complex shapes, like the vertebrae that support the spinal cord and protect it from compressive forces.
Sesamoid Bones
A sesamoid bone is a small, round bone that, as the name suggests, is shaped like a sesame seed. These bones form in tendons (the sheaths of tissue that connect bones to muscles), where a great deal of pressure is generated in a joint. The sesamoid bones protect tendons by helping them overcome compressive forces and often provide a mechanical lever to allow a muscle/tendon greater torque about a joint. Sesamoid bones vary in number and placement from person to person but are typically found in tendons associated with the feet, hands, and knees. The patellae (singular = patella) are the only sesamoid bones found in common with every person.
Pneumatized Bones
A pneumatized bone is often hollow or contains numerous air pockets. These bones tend to be in the skull and face. Pneumatized bones decrease the weight of the skull and are often sites for the sinuses. Pneumatized bones may also be cross-listed as irregular bones.
| Bone Classifications | |||
|---|---|---|---|
| Bone classification | Features | Function(s) | Examples |
| Long | Cylinder-like shape, longer than it is wide | Leverage | Femur, tibia, fibula, metatarsals, humerus, ulna, radius, metacarpals, phalanges |
| Short | Cube-like shape, approximately equal in length, width, and thickness | Provide stability, support, while allowing for some motion | Carpals, tarsals |
| Flat | Thin and curved | Points of attachment for muscles; protectors of internal organs | Sternum, ribs, scapulae, cranial bones |
| Irregular | Complex shape | Protect internal organs | Vertebrae, facial bones |
| Sesamoid | Small and round; embedded in tendons | Protect tendons from compressive forces | Patellae |
| Pneumatized | Hollow or full of air pockets | Lighten the skull, full of air pockets (sinuses) | Ethmoid, sphenoid |
Gross Anatomy of Bone
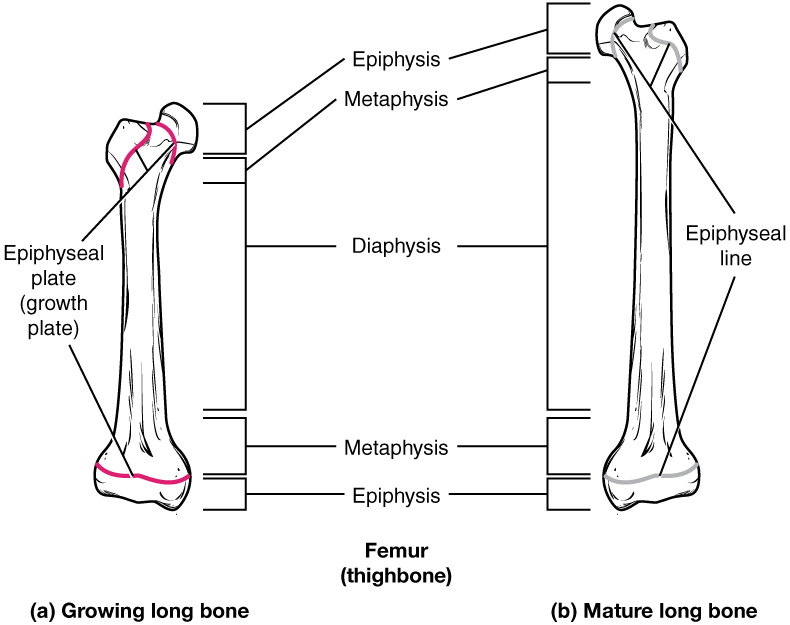
The structure of a long bone allows for the best visualization of all of the parts of a bone. A long bone has three parts: the diaphysis, the metaphysis, and the epiphysis. The diaphysis is the tubular shaft that runs between the proximal and distal ends of the bone. The hollow region in the diaphysis is called the medullary cavity, which is filled with yellow marrow. The walls of the diaphysis are composed of dense and hard compact bone.
Anatomy of a Long Bone
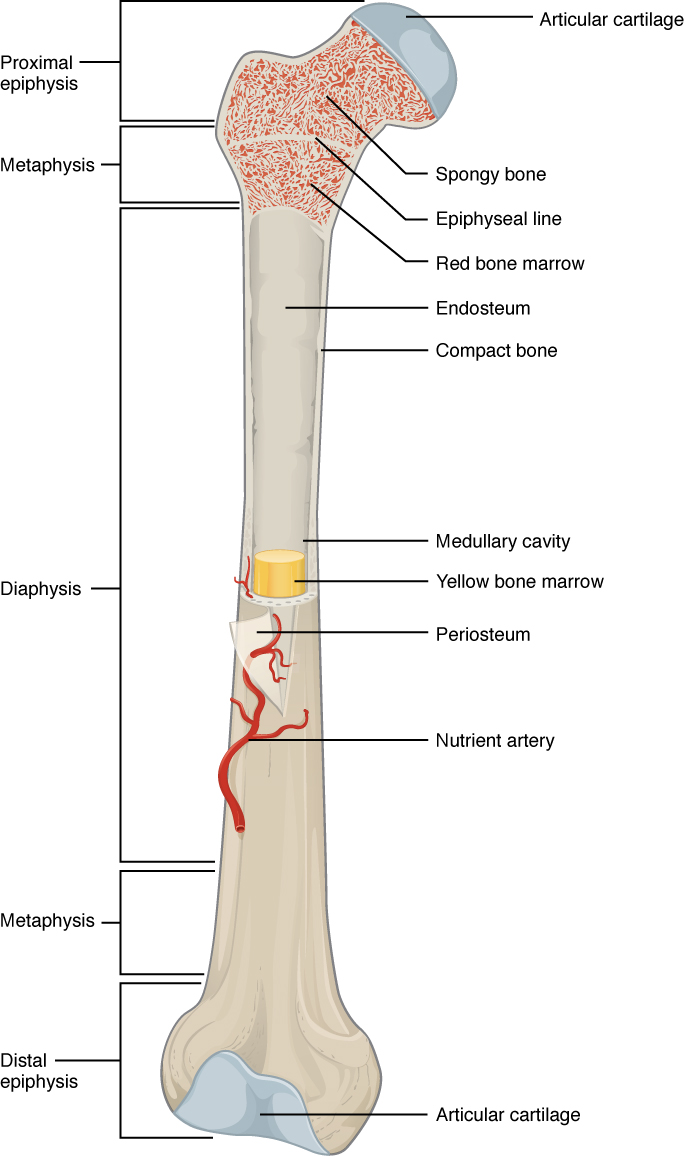
The wider section at each end of the bone is called the epiphysis (plural = epiphyses), filled with spongy bone. Red marrow fills the spaces in the spongy bone. Each epiphysis meets the diaphysis at the metaphysis, the narrow area that contains the epiphyseal plate (growth plate), a layer of hyaline cartilage in a growing bone. When the bone stops growing in early adulthood (approximately 18–21 years), the cartilage is replaced by osseous tissue, and the epiphyseal plate becomes an epiphyseal line.
The medullary cavity has a delicate membranous lining called the endosteum (end- = “inside”; oste- = “bone”), where bone growth, repair, and remodeling occur. The outer surface of the bone is covered with a fibrous membrane called the periosteum (peri– = “around” or “surrounding”). The periosteum contains blood vessels, nerves, and lymphatic vessels that nourish compact bone. Tendons and ligaments also attach to bones at the periosteum. The periosteum covers the entire outer surface except where the epiphyses meet other bones to form joints. In this region, the epiphyses are covered with articular cartilage, a thin layer of cartilage that reduces friction and acts as a shock absorber.
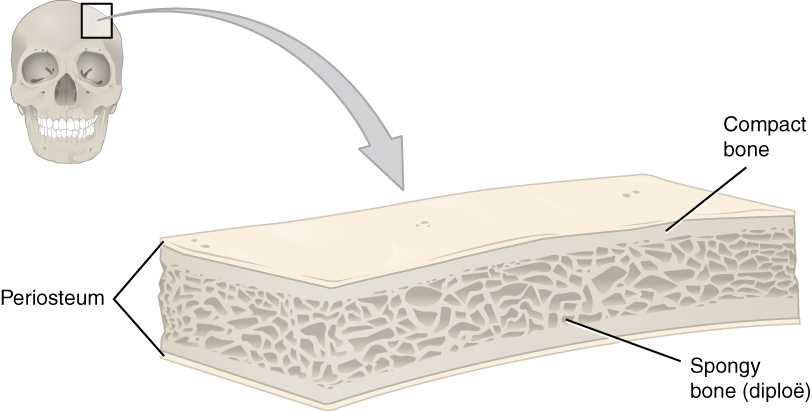
Like those of the cranium, flat bones consist of a layer of spongy bone (diploë), lined on either side by a layer of compact bone. The two layers of compact bone and the interior spongy bone work together to protect the internal organs.
Compact and Spongy Bone
The differences between compact and spongy bone are best explored via their histology. Most bones contain compact and spongy osseous tissue, but their distribution and concentration vary based on the bone’s overall function. Compact bone is dense to withstand compressive forces, while spongy (cancellous) bone has open spaces and supports shifts in weight distribution.
Compact Bone
Compact bone is the denser, stronger of the two types of bone tissue. It can be found under the periosteum and in the diaphyses of long bones, where it provides support and protection.
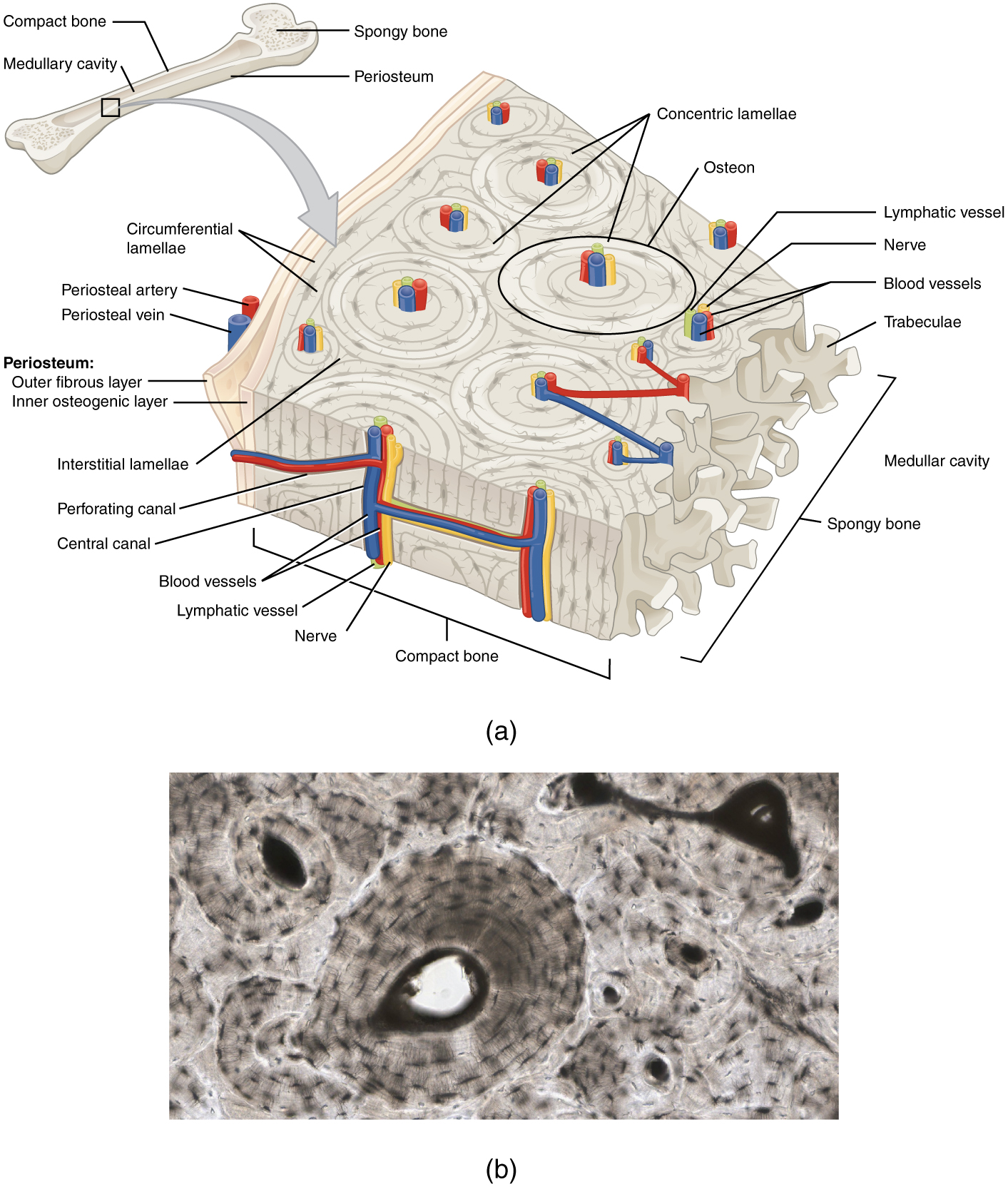
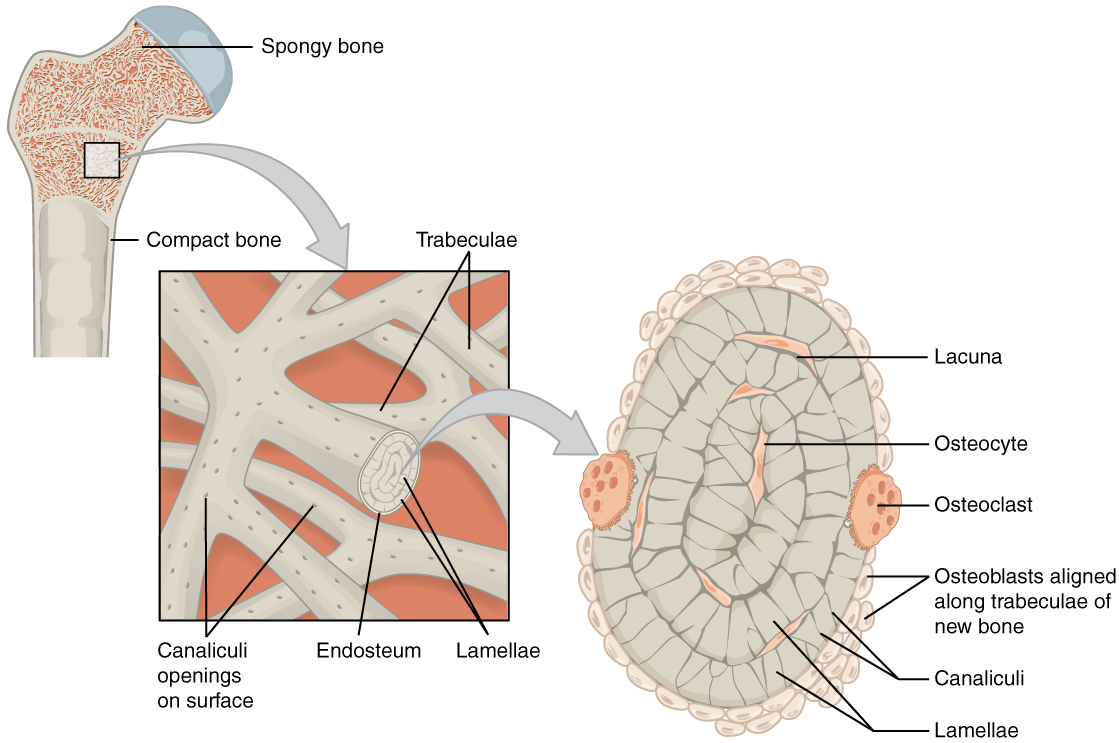
The microscopic structural unit of compact bone is called an osteon, or Haversian system. Each osteon is composed of concentric rings of calcified matrix called lamellae (singular = lamella). Running down the center of each osteon is the central canal, or Haversian canal, which contains blood vessels, nerves, and lymphatic vessels. These vessels and nerves branch off at right angles through a perforating canal, also known as Volkmann’s canals, to extend to the periosteum and endosteum.
The osteocytes are located inside spaces called lacunae (singular = lacuna), found at the borders of adjacent lamellae. Canaliculi connect with the canaliculi of other lacunae and eventually with the central canal. This system allows nutrients to be transported to the osteocytes and wastes to be removed from them.
Spongy (Cancellous) Bone
Like compact bone, spongy bone, also known as cancellous bone, contains osteocytes housed in lacunae, but they are not arranged in concentric circles. Instead, the lacunae and osteocytes are found in a lattice-like network of matrix spikes called trabeculae (singular = trabecula). The trabeculae may appear to be a random network, but each trabecula forms along the lines of stress to provide strength to the bone. The trabeculated network spaces provide balance to the dense and heavy compact bone by making bones lighter so that muscles can move them more easily. In addition, the spaces in some spongy bones contain red marrow, protected by the trabeculae, where hematopoiesis occurs.
Blood and Nerve Supply
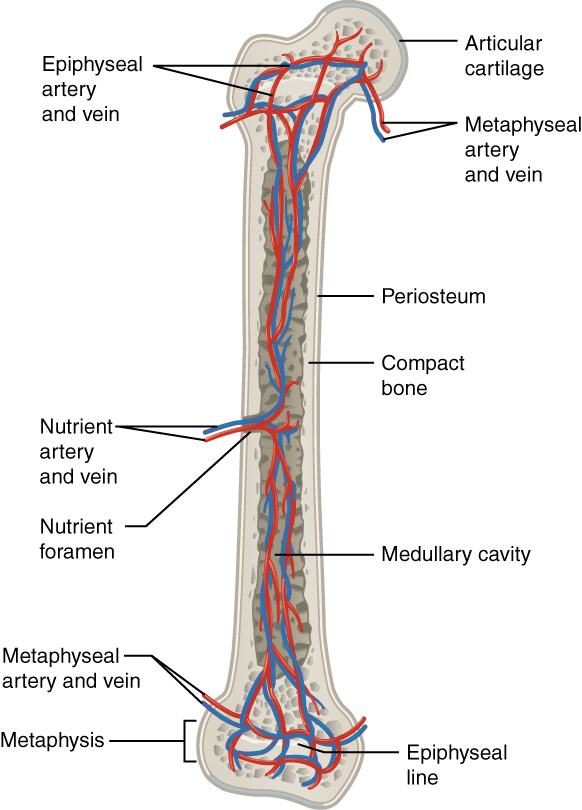
The spongy bone and medullary cavity receive nourishment from arteries that pass through the compact bone. The nutrient artery and vein that supply the diaphysis enter through the nutrient foramen (plural = foramina). The blood supplies to/from the metaphysis are called metaphyseal arteries and veins. The blood supplies to/from the epiphysis are called epiphyseal artery and vein. Finally, the blood supplies to/from the periosteum are called the periosteal arteries and veins.
The osteocytes in spongy bone are nourished by blood vessels of the periosteum that penetrate spongy bone and blood that circulates in the marrow cavities. As the blood passes through the marrow cavities, it is collected by veins, passing out of the bone through the foramina.
In addition to the blood vessels, nerves follow the same paths into the bone, where they tend to concentrate in the more metabolically active regions of the bone. The nerves sense pain, and it appears the nerves also play roles in regulating blood supplies and bone growth, hence their concentrations in the bone’s metabolically active sites.
Osteogenic Pathways
In the early stages of embryonic development, the embryo’s skeleton consists of fibrous membranes and hyaline cartilage. By the sixth or seventh week of embryonic life, the actual process of bone development, ossification (osteogenesis), begins. There are two osteogenic pathways—intramembranous ossification and endochondral ossification—but bone is the same regardless of the pathway that produces it.
Cartilage Templates
Bone is a replacement tissue; that is, it uses a model tissue to lay down its mineral matrix. For skeletal development, the most common template is cartilage. During fetal development, a framework is laid down that determines where bones will form. This framework is a flexible, semi-solid matrix produced by chondroblasts and consists of hyaluronic acid, chondroitin sulfate, collagen fibers, and water. As the matrix surrounds and isolates chondroblasts, they are called chondrocytes. Unlike most connective tissues, cartilage is avascular, meaning that it has no blood vessels supplying nutrients and removing metabolic wastes. All of these functions are carried on by diffusion through the matrix. This is why damaged cartilage does not repair itself as readily as most tissues do.
Throughout fetal development and into childhood growth and development, bone forms on the cartilaginous matrix. By the time a fetus is born, most of the cartilage has been replaced with bone. Some additional cartilage will be replaced throughout childhood, and some cartilage remains in the adult skeleton.
Intramembranous Ossification
During intramembranous ossification, compact and spongy bone develops directly from sheets of mesenchymal (undifferentiated) connective tissue. The flat bones of the face, most of the cranial bones, and the clavicles (collarbones) are formed via intramembranous ossification.
The process begins when mesenchymal cells in the embryonic skeleton gather together and differentiate into specialized cells. Some of these cells will differentiate into capillaries, while others will become osteogenic cells and then osteoblasts. Although the formation of bone tissue will ultimately spread them out, early osteoblasts appear in a cluster called an ossification center.
The osteoblasts secrete osteoid, uncalcified matrix, which calcifies (hardens) within a few days as mineral salts are deposited on it, thereby entrapping the osteoblasts within. Once entrapped, the osteoblasts become osteocytes. As osteoblasts transform into osteocytes, osteogenic cells in the surrounding connective tissue differentiate into new osteoblasts.
Osteoid (unmineralized bone matrix) secreted around the capillaries results in a trabecular matrix, while osteoblasts on the surface of the spongy bone become the periosteum. The periosteum then creates a protective layer of compact bone superficial to the trabecular bone. The trabecular bone crowds nearby blood vessels, which eventually condense into red marrow.
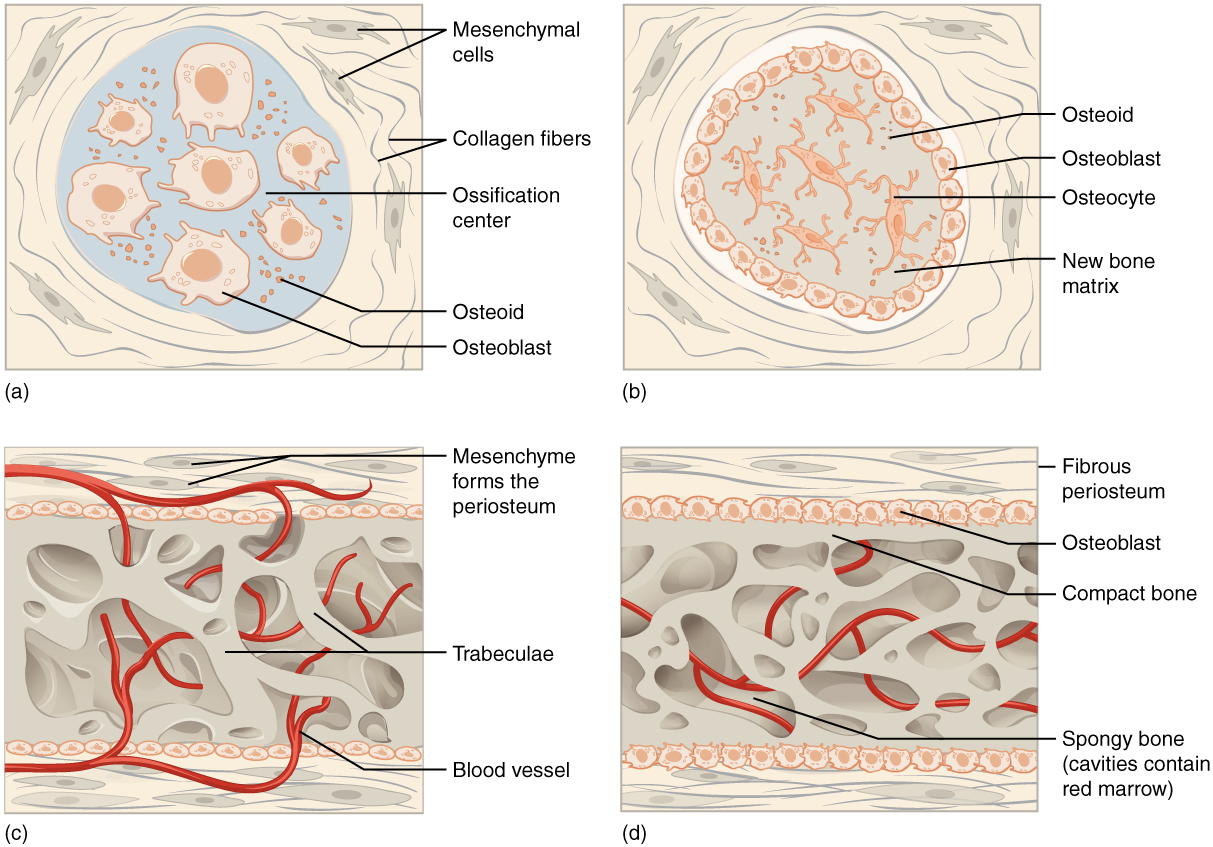
Intramembranous ossification begins in utero during fetal development and continues into adolescence. At birth, the skull and clavicles are not fully ossified, nor are the sutures of the skull closed. This allows the skull and shoulders to deform during passage through the birth canal. The last bones to ossify via intramembranous ossification are the face’s flat bones, which reach their adult size at the end of the adolescent growth spurt.
Endochondral Ossification
In endochondral ossification, bone develops by replacing hyaline cartilage. Cartilage does not become bone. Instead, cartilage serves as a template to be completely replaced by new bone. Endochondral ossification takes much longer than intramembranous ossification. Bones at the base of the skull and long bones form via endochondral ossification.
In a long bone, for example, at about 6 to 8 weeks after conception, some of the mesenchymal cells differentiate into chondrocytes (cartilage cells) that form the cartilaginous skeletal precursor of the bones. Soon after, the perichondrium, a membrane that covers the cartilage, appears.
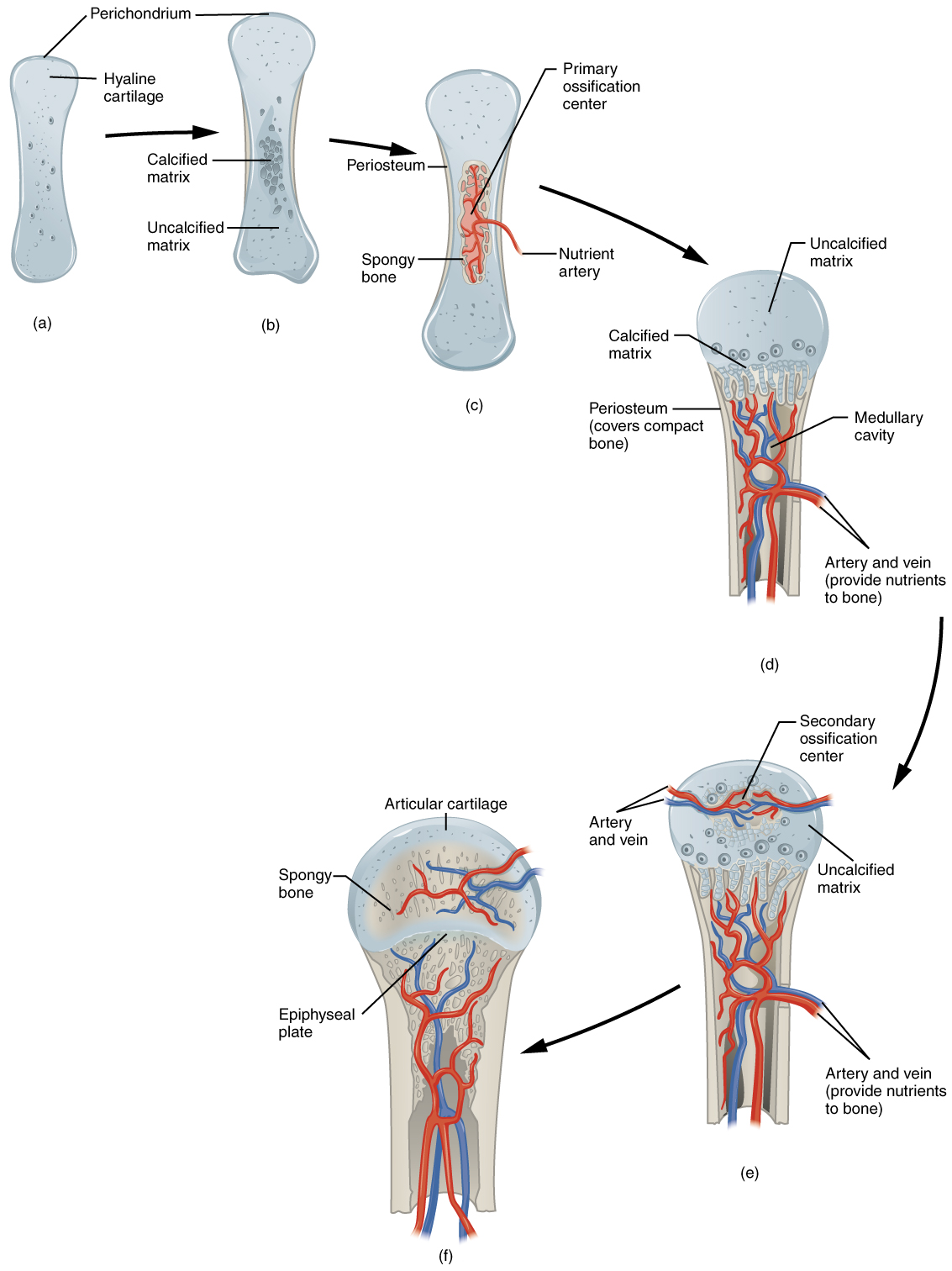
As more matrix is produced, the chondrocytes in the center of the cartilaginous model grow in size. As the matrix calcifies, nutrients can no longer reach the chondrocytes. This results in their death and the disintegration of the surrounding cartilage. Blood vessels invade the resulting spaces, not only enlarging the cavities but also carrying osteogenic cells with them, many of which will become osteoblasts. These enlarging spaces eventually combine to become the medullary cavity.
As the cartilage grows, capillaries penetrate it. This penetration initiates the transformation of the perichondrium into the bone-producing periosteum. Here, the osteoblasts form a periosteal collar of compact bone around the cartilage of the diaphysis. By the second or third month of fetal life, bone cell development and ossification ramps up and creates the primary ossification center, a region deep in the periosteal collar where ossification begins.
While these big changes are occurring, chondrocytes and cartilage continue to grow at the ends of the bone (the future epiphyses), which increases the bone’s length at the same time bone is replacing cartilage in the diaphyses. By the time the fetal skeleton is fully formed, cartilage only remains at the joint surface as articular cartilage and between the diaphysis and epiphysis as the epiphyseal plate, the latter of which is responsible for the longitudinal growth of bones. After birth, this same sequence of events (matrix mineralization, death of chondrocytes, invasion of blood vessels from the periosteum, and seeding with osteogenic cells that become osteoblasts) occurs in the epiphyseal regions, and each of these centers of activity is referred to as a secondary ossification center.
How Bones Grow in Length (Interstitial Bone Growth)
The epiphyseal plate is the area of growth in a long bone. It is a layer of hyaline cartilage where ossification occurs in immature bones. On the epiphyseal side of the epiphyseal plate, cartilage is formed. On the diaphyseal side, cartilage is ossified, and the diaphysis grows in length. The epiphyseal plate is composed of four zones of cells and activity. The reserve zone is the region closest to the epiphyseal end of the plate and contains small chondrocytes within the matrix. These chondrocytes do not participate in bone growth but secure the epiphyseal plate to the osseous tissue of the epiphysis.
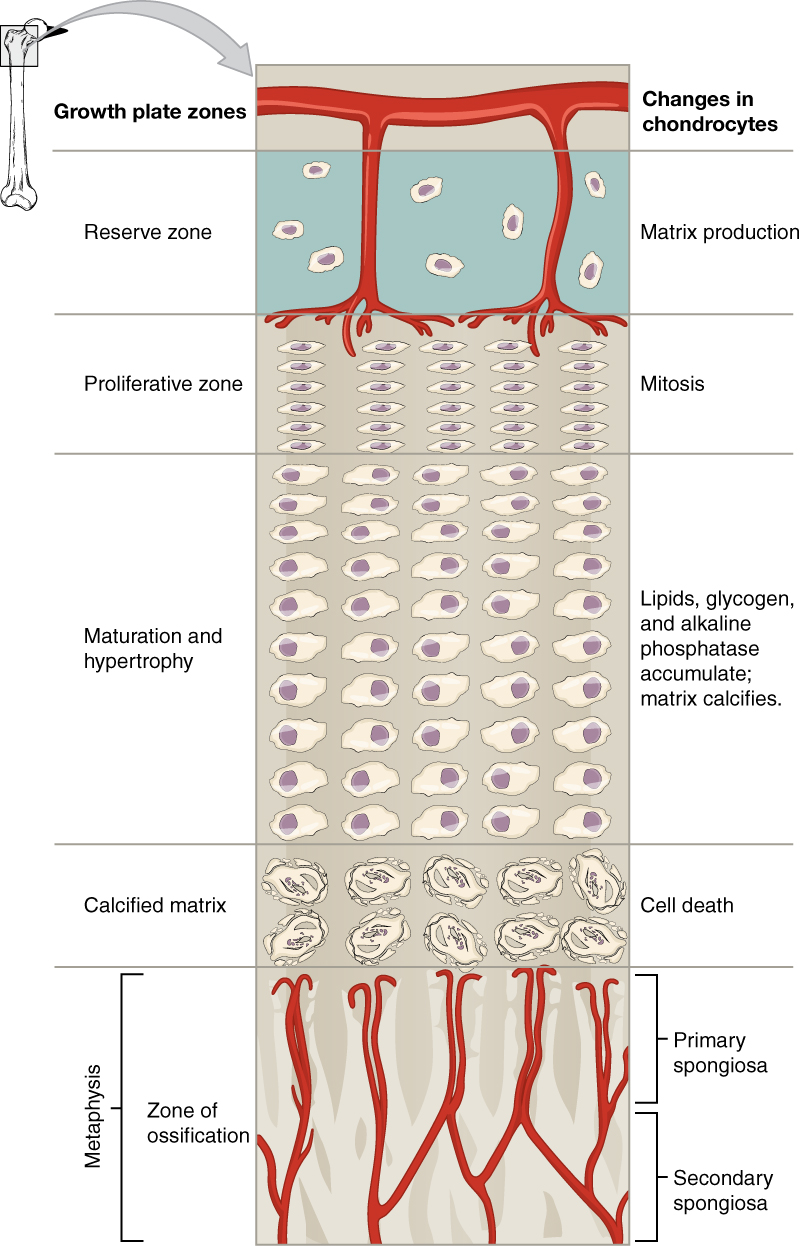
The proliferative zone is the next layer toward the diaphysis and contains stacks of slightly larger chondrocytes. It makes new chondrocytes (via mitosis) to replace those that die at the diaphyseal end of the plate. Chondrocytes in the next layer, the zone of maturation and hypertrophy, are older and larger than those in the proliferative zone. The more mature cells are situated closer to the diaphyseal end of the plate. The longitudinal growth of bone results from cellular division in the proliferative zone and the maturation of cells in the zone of maturation and hypertrophy.
Most of the chondrocytes in the zone of calcified matrix, the zone closest to the diaphysis, are dead because the matrix around them has calcified. Capillaries and osteoblasts from the diaphysis penetrate this zone, and the osteoblasts secrete bone tissue on the remaining calcified cartilage. Thus, the zone of calcified matrix connects the epiphyseal plate to the diaphysis. A bone grows in length when osseous tissue is added to the diaphysis.
Bones continue to grow in length until early adulthood. Hormones control the rate of growth. When the chondrocytes in the epiphyseal plate cease their proliferation and bone replaces the cartilage, longitudinal growth stops. All that remains of the epiphyseal plate is the epiphyseal line.
How Bones Grow in Diameter (Appositional Bone Growth)
While bones are increasing in length, they are also increasing in diameter; growth in diameter can continue even after longitudinal growth ceases. This is called appositional growth. Osteoclasts resorb old bone that lines the medullary cavity, while osteoblasts, via intramembranous ossification, produce new bone tissue beneath the periosteum. The erosion of old bone along the medullary cavity and the deposition of new bone beneath the periosteum increase the diameter of the diaphysis and increase the diameter of the medullary cavity. This process is called modeling.
Exercise and Bone Tissue
During long space missions, astronauts can lose approximately 1 to 2 percent of their bone mass per month. This loss of bone mass is thought to be caused by the lack of mechanical stress on astronauts’ bones due to the low gravitational forces in space. Lack of mechanical stress causes bones to lose mineral salts and collagen fibers, and thus strength. Similarly, mechanical stress stimulates the deposition of mineral salts and collagen fibers. The internal and external structure of a bone will change as stress increases or decreases so that the bone is an ideal size and weight for the amount of activity it endures. That is why people who exercise regularly have thicker bones than people who are more sedentary. It is also why a broken bone in a cast atrophies while its contralateral mate maintains its mineral salts and collagen fibers’ concentration. The bones undergo remodeling as a result of forces (or lack of forces) placed on them.
Numerous controlled studies have demonstrated that people who exercise regularly have greater bone density than those who are more sedentary. Any type of exercise will stimulate more bone tissue deposition, but resistance training has a greater effect than cardiovascular activities. Resistance training is essential to slow down the eventual bone loss due to aging and preventing osteoporosis.
Nutrition and Bone Tissue
The vitamins and minerals in all of the food we consume are important for all of our organ systems. However, certain nutrients affect bone health.
Fun Fact – Calcium and Vitamin D (-> you will not be assessed on this content)
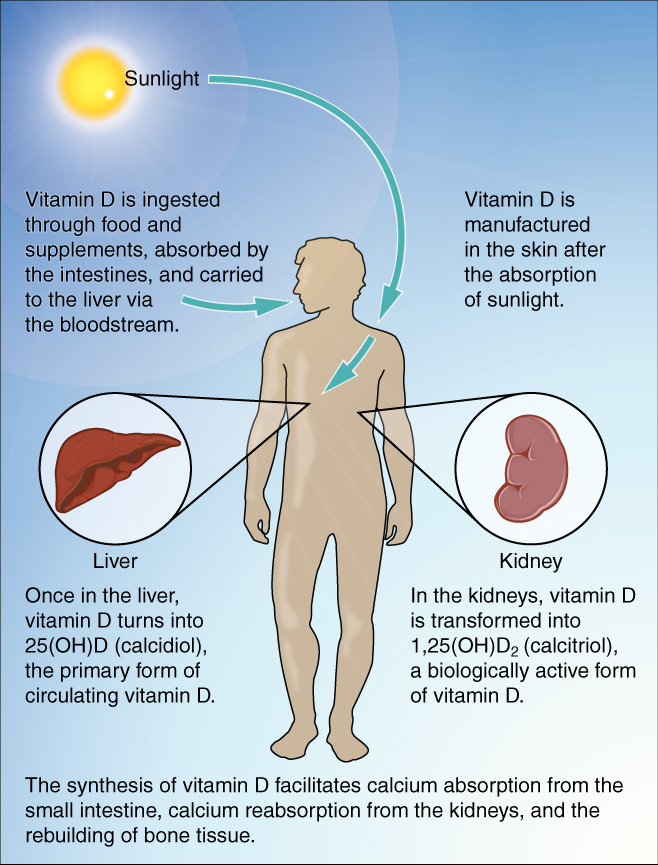
You already know that calcium is a critical component of bone, especially in calcium phosphate and calcium carbonate. Since the body cannot make calcium, it must be obtained from the diet. However, calcium cannot be absorbed from the small intestine without vitamin D. Therefore, vitamin D intake is also critical to bone health.
Milk and other dairy foods are not the only sources of calcium. This important nutrient is also found in green leafy vegetables, broccoli, nuts, beans, seeds, and shellfish in smaller quantities.
The action of sunlight on the skin triggers the body to produce its own vitamin D. Still, many people, especially those of darker complexion and those living in northern latitudes where the sun’s rays are not as strong, are deficient in vitamin D. In cases of deficiency, a doctor can prescribe a vitamin D supplement.
Fun Fact – Other nutrients supporting bone health (-> you will not be assessed on this content)
Vitamin K also supports bone mineralization and may have a synergistic role with vitamin D in the regulation of bone growth. Green leafy vegetables are a good source of vitamin K.
The minerals magnesium and fluoride may also play a role in supporting bone health. While magnesium is only found in trace amounts in the human body, more than 60 percent of it is in the skeleton, suggesting it plays a role in bone structure. Fluoride can displace the hydroxyl group in bone’s hydroxyapatite crystals and form fluorapatite. Similar to its effect on dental enamel, fluorapatite helps stabilize and strengthen bone mineral. Fluoride can also enter spaces within hydroxyapatite crystals, thus increasing their density.
Omega-3 fatty acids have long been known to reduce inflammation in various parts of the body. Inflammation can interfere with osteoblasts’ function, so consuming omega-3 fatty acids in the diet or supplements may also help enhance new osseous tissue production.
| Nutrients and Bone Health | |
|---|---|
| Nutrient | Role in bone health |
| Calcium | Needed to make calcium phosphate and calcium carbonate, which form the hydroxyapatite crystals that give bone its hardness |
| Vitamin D | Needed for calcium absorption |
| Vitamin K | Supports bone mineralization; may have synergistic effect with vitamin D |
| Magnesium | Structural component of bone |
| Fluoride | Structural component of bone |
| Omega-3 fatty acids | Reduces inflammation that may interfere with osteoblast function |
Hormones and Bone Tissue
The endocrine system produces and secretes hormones, many of which interact with the skeletal system. These hormones control bone growth, maintaining bone once it is formed, and remodeling it. The hormones that influence osteoblasts are growth hormone, thyroxine, calcitriol, and sex hormones (estrogen and testosterone). Hormones that influence osteoclasts include parathyroid hormone and calcitonin.
| Hormones That Affect the Skeletal System | |
|---|---|
| Hormone | Role |
| Growth hormone | Increases length of long bones, enhances mineralization, and improves bone density |
| Thyroxine | Stimulates bone growth and promotes synthesis of bone matrix |
| Sex hormones | Promote osteoblastic activity and production of bone matrix; responsible for adolescent growth spurt; promote conversion of epiphyseal plate to epiphyseal line |
| Calcitriol | Stimulates absorption of calcium and phosphate from digestive tract |
| Parathyroid hormone | Stimulates osteoclast proliferation and resorption of bone by osteoclasts; promotes reabsorption of calcium by kidney tubules; indirectly increases calcium absorption by small intestine |
| Calcitonin | Inhibits osteoclast activity and stimulates calcium uptake by bones |
Aging and the Skeletal System
Osteoporosis is a disease characterized by a decrease in bone mass that occurs when the rate of bone resorption exceeds the rate of bone formation, a common occurrence as the body ages. While osteoporosis can involve any bone, it most commonly affects the femur’s proximal ends, vertebrae, and wrist. As a result of bone density loss, the osseous tissue may not provide adequate support for everyday functions. Histologically, osteoporosis is characterized by a reduction in compact bone thickness and the number and size of trabeculae in cancellous bone.
Bone Loss – FYI (For Your Information -> you will not be assessed on this content)
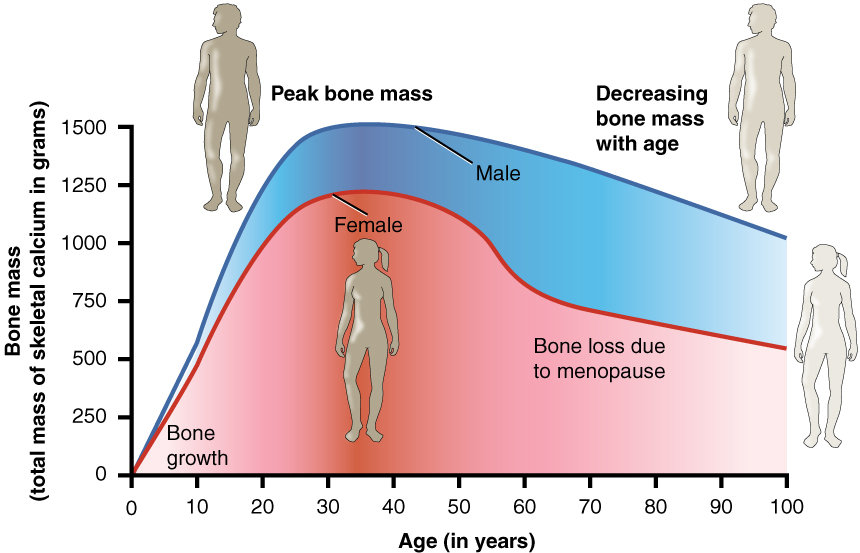
Women lose bone mass more quickly than men starting at about 50 years of age. This occurs because 50 is the approximate age at which women go through menopause. Not only do their menstrual periods lessen and eventually cease, but their ovaries reduce in size and then cease the production of estrogen, a hormone that promotes osteoblastic activity and production of bone matrix. Thus, osteoporosis is more common in women than in men, but men can also develop it. Anyone with a family history of osteoporosis has a greater risk of developing the disease, so the best treatment is prevention, which should start with a childhood diet that includes adequate calcium and vitamin D intake and a lifestyle that includes weight-bearing exercise. These actions, as discussed above, are important in building bone mass. Promoting proper nutrition and weight-bearing exercise early in life can maximize bone mass before 30, thus reducing the risk of osteoporosis.
For many older people, a hip fracture can be life-threatening. The fracture itself may not be serious, but the immobility that comes during the healing process can lead to the formation of blood clots that can lodge in the capillaries of the lungs, resulting in respiratory failure; pneumonia due to the lack of poor air exchange that accompanies immobility; pressure sores (bed sores) that allow pathogens to enter the body and cause infections; and urinary tract infections from catheterization.
Current treatments for managing osteoporosis include bisphosphonates (the same medications often used in Paget’s disease), calcitonin, and estrogen (for women only). Minimizing the risk of falls, for example, by removing tripping hazards, is also an important step in managing the disease’s potential outcomes.

