Additional Reading Materials
Chapter 4: Covalent Bond and Lewis Structure
Ch4.1 Covalent Bond
In ionic compounds, electrons are transferred between atoms of different elements to form ions. But this is not the only way that compounds can be formed. Atoms can also make chemical bonds by sharing electrons between each other. Such bonds are called covalent bonds. Covalent bonds are formed between two atoms when both have similar tendencies to attract electrons to themselves (i.e., when both atoms have fairly similar ionization energies and electron affinities). For example, two hydrogen atoms bond covalently to form an H2 molecule; each hydrogen atom in the H2 molecule has two electrons stabilizing it, giving each atom the same number of valence electrons as the noble gas He.
Compounds that contain covalent bonds exhibit different physical properties than ionic compounds. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds. In fact, many covalent compounds are liquids or gases at room temperature, and, in their solid states, they are typically much softer than ionic solids. Furthermore, whereas ionic compounds are good conductors of electricity when dissolved in water, most covalent compounds are insoluble in water; since they are electrically neutral, they are poor conductors of electricity in any state.
Formation of Covalent Bonds
Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. For example, the hydrogen molecule, H2, contains a covalent bond between its two hydrogen atoms. Figure 1 illustrates why this bond is formed. Starting on the far right, we have two separate hydrogen atoms with a particular potential energy, indicated by the red line. Along the x-axis is the distance between the two atoms. As the two atoms approach each other (moving left along the x-axis), their valence orbitals (1s) begin to overlap. The single electrons on each hydrogen atom then interact with both atomic nuclei, occupying the space around both atoms. The strong attraction of each shared electron to both nuclei stabilizes the system, and the potential energy decreases as the bond distance decreases. If the atoms continue to approach each other, the positive charges in the two nuclei begin to repel each other, and the potential energy increases. The bond length is determined by the distance at which the lowest potential energy is achieved.
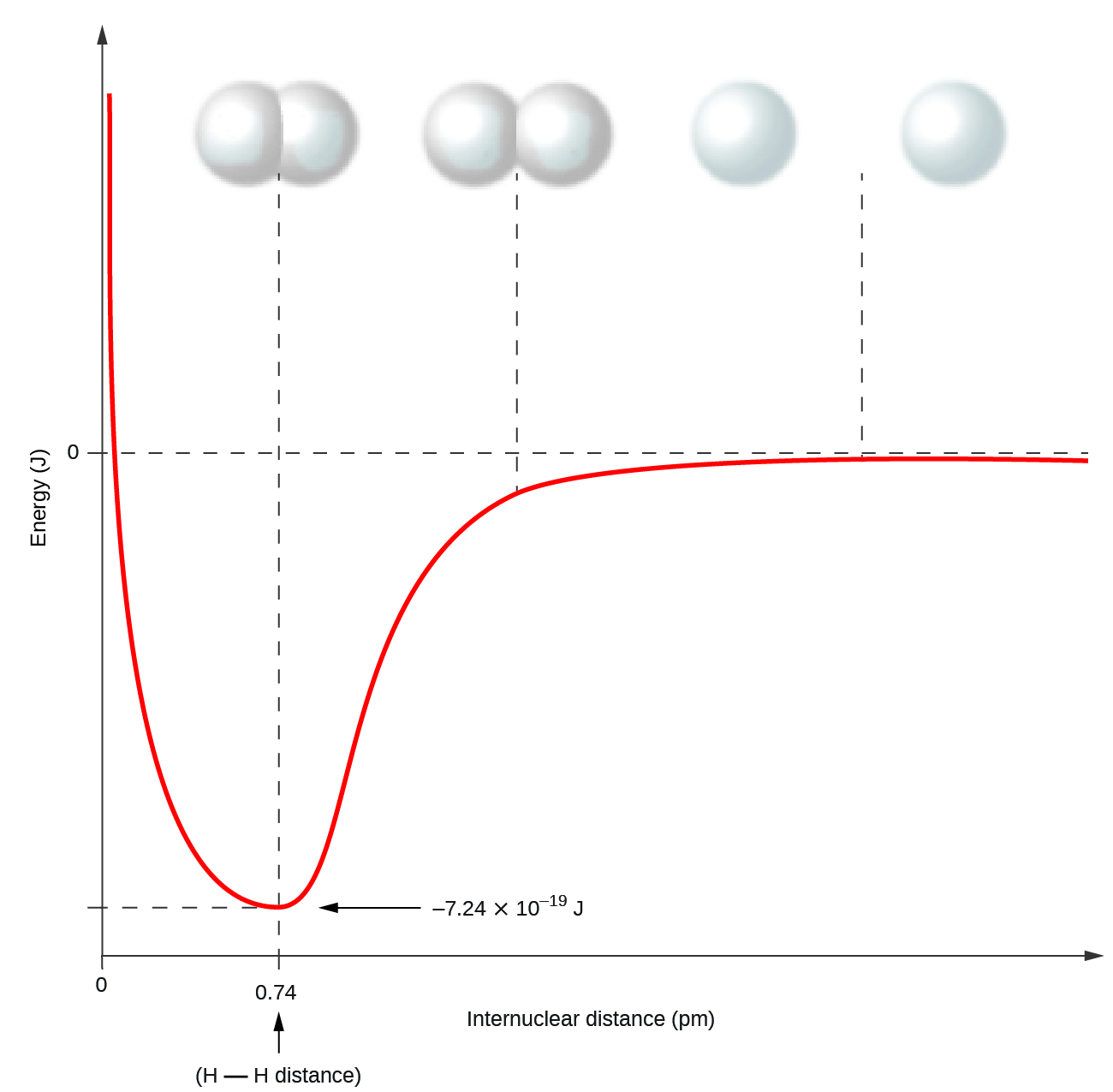
It is essential to remember that energy must be added to break chemical bonds (an endothermic process), whereas forming chemical bonds releases energy (an exothermic process). In the case of H2, the covalent bond is very strong; a large amount of energy, 436 kJ, must be added to break the bonds in one mole of hydrogen molecules and cause the atoms to separate:
Conversely, the same amount of energy is released when one mole of H2 molecules forms from two moles of H atoms:
Ch4.2 Molecular Orbital Theory
Molecular orbital (MO) theory describes the distribution of electrons in a molecule in much the same way that the distribution of electrons in an atom is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is also described by a wave function, Ψ, analogous to the behavior in an atom. Just like electrons around isolated nucleus, electrons around nucleus in molecules are limited to discrete (quantized) energies. The region of space in which an electron in a molecule is likely to be found is called a molecular orbital. Like an atomic orbital, a single molecular orbital is full when it contains two electrons with opposite spin.
We will first consider the molecular orbitals in molecules composed of two identical atoms (H2 or Cl2, for example). Such molecules are called homonuclear diatomic molecules. In these diatomic molecules, several types of molecular orbitals occur.
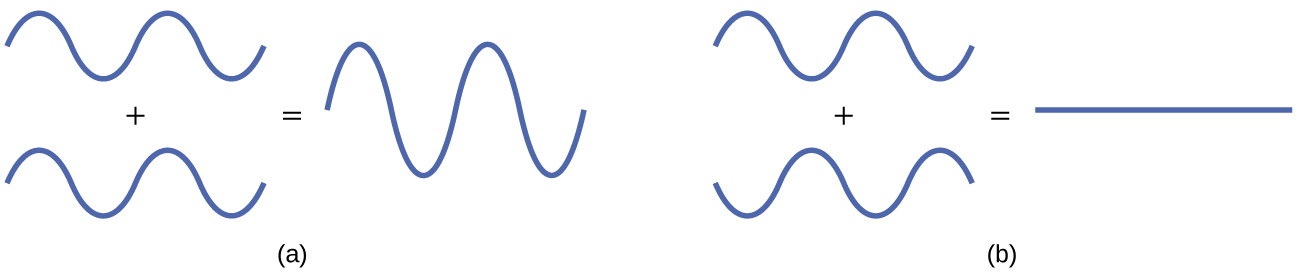
The mathematical process of combining atomic orbitals to generate molecular orbitals is called the linear combination of atomic orbitals (LCAO). The wave function describes the wavelike properties of an electron. Molecular orbitals are combinations of atomic orbital wave functions. Combining waves can lead to constructive interference, in which peaks line up with peaks, or destructive interference, in which peaks line up with troughs (Figure 2). In orbitals, the waves are three dimensional, and they can combine in-phase, producing regions with a higher probability of electron density, or out-of-phase, producing nodes or regions of low electron density.
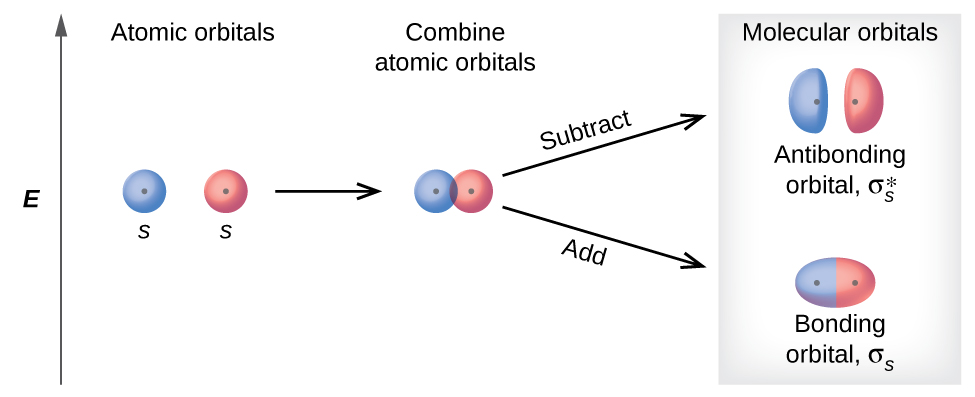
There are two types of molecular orbitals that can form from the overlap of two atomic s orbitals on adjacent atoms, illustrated in Figure 3. The in-phase combination produces a σs molecular orbital (read as "sigma-s") in which most of the electron density is located between the nuclei and are attracted by both nuclei at the same time. The σs MO is more stable (lower energy) than the individual atom orbitals and contribute to the formation of a covalent bond. Therefore we call these bonding orbitals. The out-of-phase combination (which can also be thought of as subtracting the wave functions) produces a σs* molecular orbital (read as "sigma-s-star") in which the electron density is located well away from the region between the two nuclei, and there is a node between the nuclei. The σs* MO is higher in energy than the individual atom orbitals and thus makes a negative contribution to bonding. These orbitals are called antibonding orbitals, denoted by the asterisk.
You can watch animations visualizing the calculated atomic orbitals combining to form various molecular orbitals at the Orbitron website.
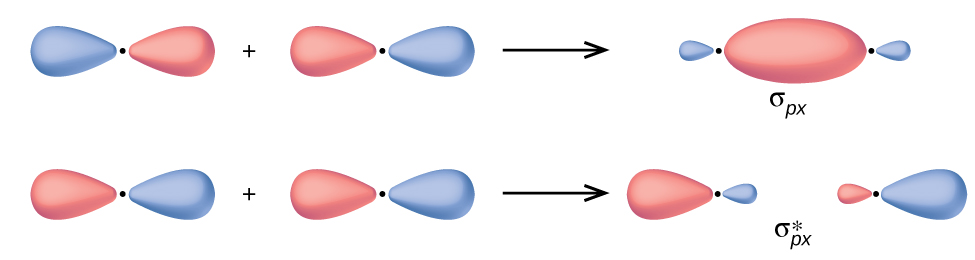
In p atomic orbitals, the wave function gives rise to two lobes with opposite phases, analogous to how a two-dimensional wave has both parts above and below the average. We indicate the phases by shading the orbital lobes differently or by using different colors. When orbital lobes of the same phase overlap, constructive interference increases the electron density. When regions of opposite phase overlap, the destructive interference decreases electron density and creates nodes. When p orbitals overlap end to end, they create σp and σp* molecular orbitals (Figure 4). If two atoms are located along the x-axis in a Cartesian coordinate system, the two px orbitals overlap end to end and form σpx (bonding) and [latex]\sigma^*_{px}[/latex] (antibonding) molecular orbitals. Just as with s-orbital overlap, the asterisk indicates the MO with antibonding characteristic that is higher in energy than the atomic p orbital used to form it.
The side-by-side overlap of two atomic p orbitals gives rise to a π and a π* molecular orbitals, as shown in Figure 5. The π MO contains a nodal plane along the internuclear axis and perpendicular to the lobes of the p orbitals, with electron density on either side of the node. This nodal plane connects the node that was present in the atomic p orbitals. An electron in the π MO interacts with both nuclei and has an energy lower than it would in the atomic p orbital, making this a bonding MO. The π* MO has an additional perpendicular nodal plane between the nuclei. It is higher in energy than the atomic p orbital and has antibonding characteristic.
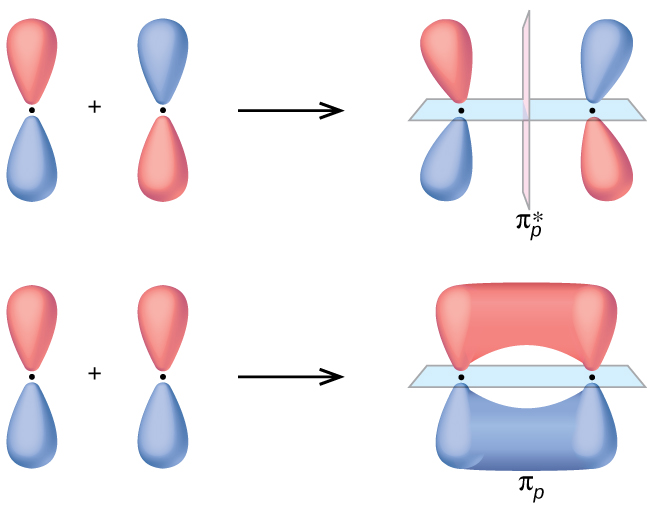
Of the three atomic p orbitals, two would orient side by side (py and pz), which can combine to create π and π* molecular orbitals. The πpy and [latex]\pi^*_{py}[/latex] MOs are oriented perpendicular to the πpz and [latex]\pi^*_{pz}[/latex] MOs, just as the atomic py and pz orbitals are perpendicular to each other. Except for their orientation, the πpy and πpz MOs are identical and have the same energy; they are degenerate orbitals. The [latex]\pi^*_{py}[/latex] and [latex]\pi^*_{px}[/latex] antibonding orbitals are also degenerate and identical except for their orientations.
Example 1
Molecular Orbitals
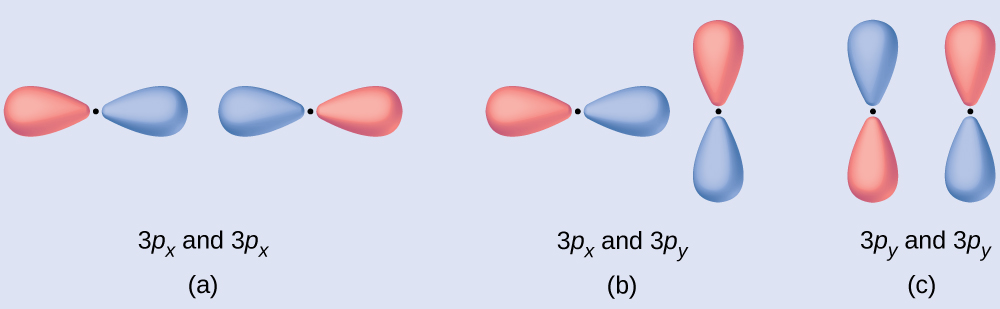
Predict what type of molecular orbital (if any) would result from combination of atomic orbitals as shown. The red and blue colors here denote the different phases in the atomic p orbital.
Solution
(a) is an in-phase combination, resulting in a σ3p molecular orbital.
(b) will not result in a molecular orbital because the constructive and destructive interference would cancel each other out (the in-phase and out-of-phase component of one atomic orbital would overlap with the same lobe on the other atomic orbital). Only orbitals with the correct alignment can combine.
(c) is an out-of-phase combination, resulting in a [latex]\pi^*_{3p}[/latex] orbital.
Check Your Learning
Label the molecular orbital shown as σ or π, bonding or antibonding and indicate where the node occurs.

Answer:
The orbital is located along the internuclear axis, so it is a σ orbital. There is a node bisecting the internuclear axis, so it is an antibonding orbital.

Walter Kohn: Nobel Laureate
Walter Kohn (Figure 6) is a theoretical physicist who studies the electronic structure of solids. His work combines the principles of quantum mechanics with advanced mathematical techniques. This technique, called density functional theory, makes it possible to compute properties of molecular orbitals, including their shape and energies. Kohn and mathematician John Pople were awarded the Nobel Prize in Chemistry in 1998 for their contributions to our understanding of electronic structure. Kohn also made significant contributions to the physics of semiconductors.

Kohn’s biography has been remarkable outside the realm of physical chemistry as well. He was born in Austria, and during World War II he was part of the Kindertransport program that rescued 10,000 children from the Nazi regime. His summer jobs included discovering gold deposits in Canada and helping Polaroid explain how its instant film worked.
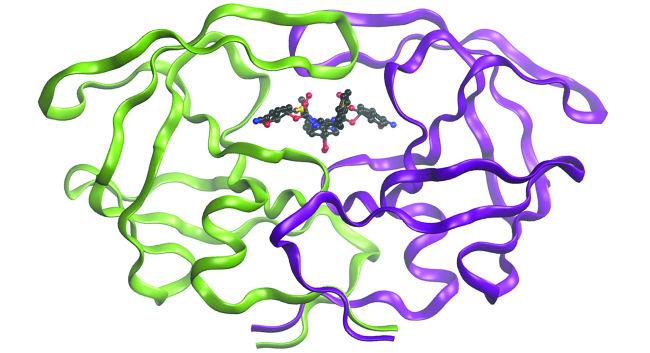
Computational Chemistry in Drug Design
While the descriptions of bonding described in this chapter involve many theoretical concepts, they also have many practical, real-world applications. For example, drug design is an important field that uses our understanding of chemical bonding to develop pharmaceuticals. This interdisciplinary area of study uses biology (understanding diseases and how they operate) to identify specific targets, such as a binding site that is involved in a disease pathway. By modeling the structures of the binding site and potential drugs, computational chemists can predict which structures can fit together and how effectively they will bind (see Figure 7). Thousands of potential candidates can be narrowed down to a few of the most promising candidates. These candidate molecules are then carefully tested to determine side effects, how effectively they can be transported through the body, and other factors. Dozens of important new pharmaceuticals have been discovered with the aid of computational chemistry, and new research projects are underway.
Ch4.3 Molecular Orbital Energy Diagram and Bond Order
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the center. Dashed lines show which of the atomic orbitals combined to form the molecular orbitals. For homonuclear diatomic molecules, each pair of atomic orbitals that combine would form one lower-energy (bonding) and one higher-energy (antibonding) molecular orbital. Thus we can see that combining the six 2p atomic orbitals results in three bonding orbitals (one σ and two π) and three antibonding orbitals (one σ* and two π*).
We predict the electron occupation in these molecular orbitals by filling them in the same way that we fill atomic orbitals, by the Aufbau principle. Lower-energy orbitals fill first, electrons spread out among degenerate orbitals before pairing up, and each molecular orbital can hold a maximum of two electrons with opposite spins. Figure 8 shows the example of Be2+.
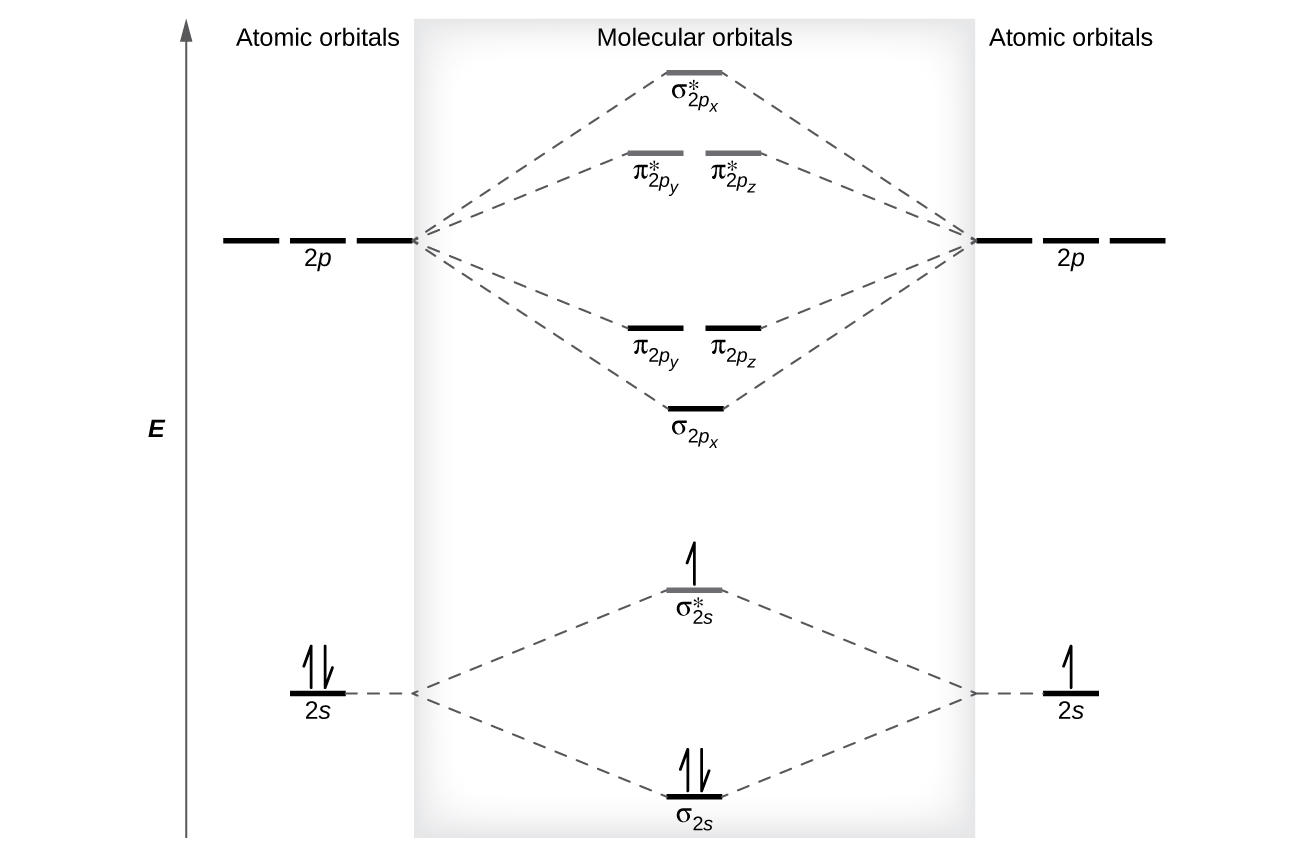
Just as we write electron configurations for atoms, we can write the molecular electronic configuration by listing the orbitals with superscripts indicating the number of electrons present. For Be2+, it would be [latex](\sigma_{1s})^2 (\sigma^*_{1s})^2 (\sigma_{2s})^2 (\sigma^*_{2s})^1[/latex]. For clarity, we place parentheses around molecular orbitals with the same energy. For simplicity, it is common to omit the core electrons from molecular orbital diagrams and include only the valence electrons.
The net contribution of the electrons to the bond strength of a molecule is identified by determining the bond order that results from the occupation of the molecular orbitals by electrons. Generally, a single bond has a bond order of 1, a double bond has a bond order of 2, and a triple bond has a bond order of 3.
An electron contributes to a bonding interaction if it occupies a bonding orbital and it contributes to an antibonding interaction if it occupies an antibonding orbital. The bond order is calculated by subtracting the destabilizing (antibonding) electrons from the stabilizing (bonding) electrons. Since a bond consists of two electrons, we divide by two to get the bond order. We can determine bond order with the following equation:
The order of a covalent bond is a guide to its strength; a bond between two atoms is stronger if the bond order is higher. We next look at some specific examples of MO diagrams and bond orders.
Ch4.4 Bonding in Diatomic Molecules
Diatomic Molecules of the First Period
In H2, the AOs of the two atoms combine to form the the σ1s and [latex]\sigma^*_{1s}[/latex] MOs.(Figure 9) The two electrons occupy the lowest energy σ1s bonding MO. From this picture, we know that H2 would readily form because the energy of a H2 molecule is lower than that of two separate H atoms (the σ1s MO is lower in energy than the two 1s atomic orbitals). The electron configuration of H2 is (σ1s)2. 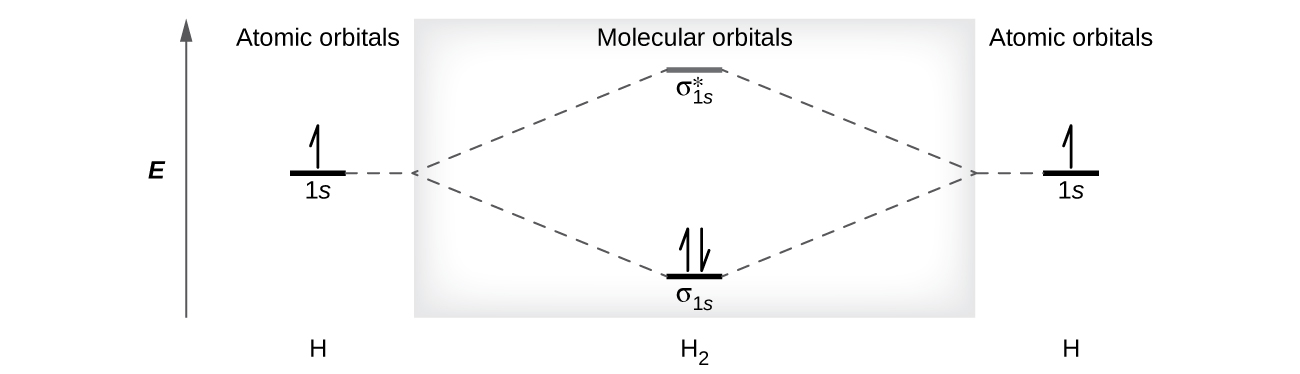
A H2 molecule contains two bonding electrons and no antibonding electrons. so it has:
This bond is a single bond.
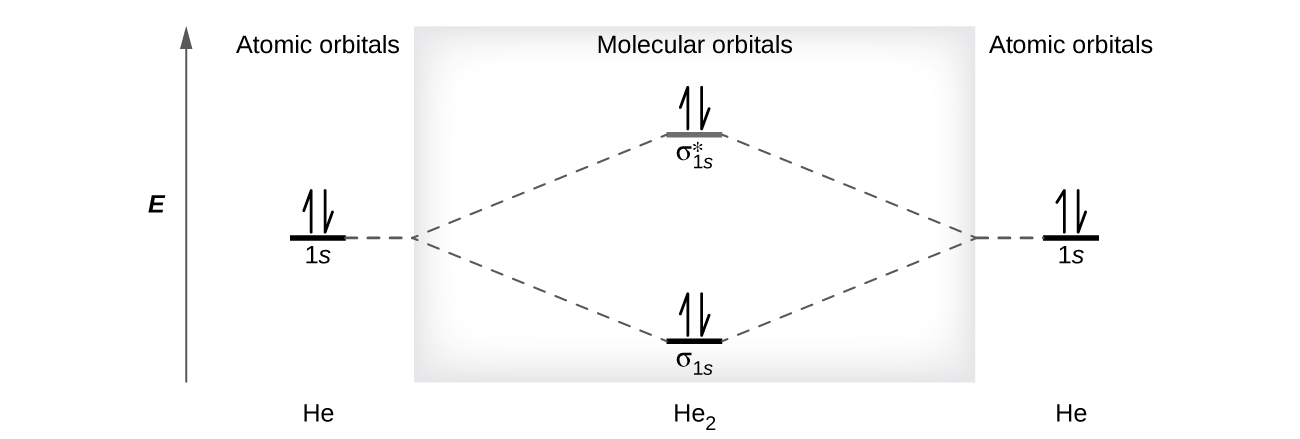
Figure 10 shows the MO diagram of a He2 molecule. There are four electrons in this system, two occupies the σ1s MO and two occupies the [latex]\sigma^*_{1s}[/latex] MO. The bond order would be:
A bond order of zero indicates that no bond is formed between two atoms. In other words, the stabilizing effect of the two electrons in the lower-energy bonding MO is offset by the destabilizing effect of the two electrons in the higher-energy antibonding MO, so there is no driving force for helium atoms to form a diatomic molecule. In fact, helium exists as discrete atoms rather than as diatomic molecules.
Diatomic Molecules of the Second Period
Eight possible homonuclear diatomic molecules might be formed by the atoms of the second period of the periodic table: Li2, Be2, B2, C2, N2, O2, F2, and Ne2. The MOs for the valence orbitals of these molecules are shown in Figure 11.
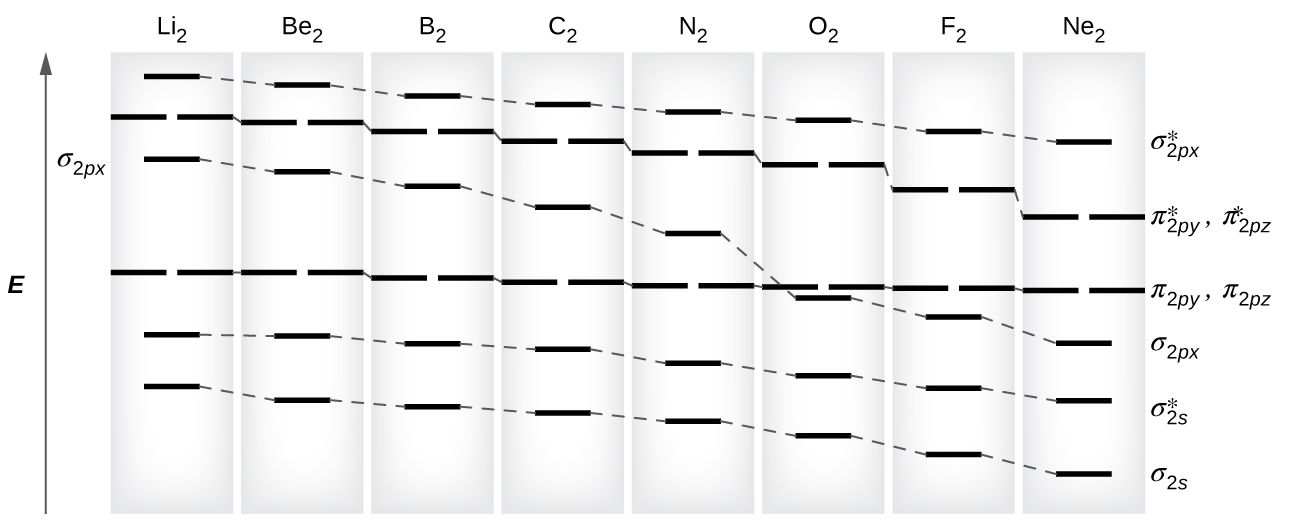
In molecular orbital theory, σ orbitals are usually more stable than π orbitals formed from degenerate atomic orbitals. However, this is not always the case. Looking at Ne2, we see that the energetic ordering of the MOs is consistent with the generic ordering. However, for atoms with three or fewer electrons in the 2p orbitals (Li through N) we observe a different pattern, in which the σ2p orbital is higher in energy than the π2p.
You can practice labeling and filling molecular orbitals with this interactive tutorial from the University of Sydney.
This switch in orbital ordering occurs because of a phenomenon called s-p mixing. s-p mixing does not create new orbitals; it merely influences the energies of the existing molecular orbitals. The σ2s wavefunction mathematically combines with the σ2p wavefunction, resulting in the σ2s MO becoming more stable and the σ2p MO becoming less stable (Figure 12). Similarly, the antibonding orbitals also undergo s-p mixing, with the σs* becoming more stable and the σp* becoming less stable.
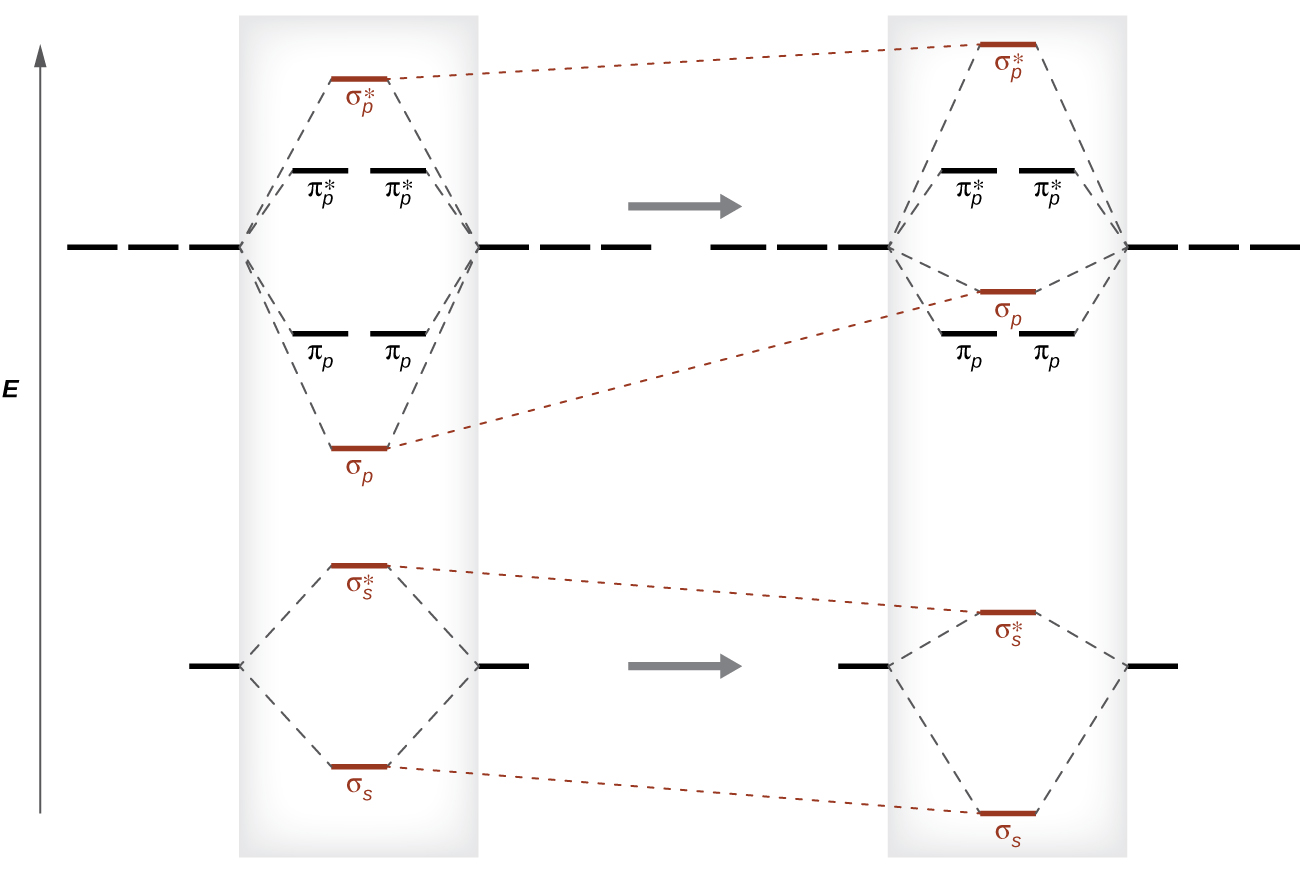
s-p mixing occurs when the s and p AOs have similar energies. When a 2p AO contains a pair of electrons, the pairing raises the energy of the orbital. Thus the 2p orbitals for O, F, and Ne are higher in energy than the 2p orbitals for Li, Be, B, C, and N. Hence, O2, F2, and N2 have negligible s-p mixing (not sufficient to change the energy ordering), and the other second period homonuclear diatomic molecules have more s-p mixing that leads to the σ2p raised above π2p.
Using the MO diagrams shown in Figure 11, we can fill in the electrons and determine the molecular electron configuration and bond order for each of the diatomic molecules. As shown in Table 1, Be2 and Ne2 molecules would have a bond order of 0, and these molecules do not exist.
| Molecule | Electron Configuration | Bond Order |
|---|---|---|
| Li2 | [latex](\sigma_{2s})^2[/latex] | 1 |
| Be2 (unstable) | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2[/latex] | 0 |
| B2 | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\pi_{2py}, \pi_{2pz})^2[/latex] | 1 |
| C2 | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\pi_{2py}, \pi_{2pz})^4[/latex] | 2 |
| N2 | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\pi_{2py}, \pi_{2pz})^4 (\sigma_{2px})^2[/latex] | 3 |
| O2 | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\sigma_{2px})^2 (\pi_{2py}, \pi_{2pz})^4 (\pi^*_{2py}, \pi^*_{2pz})^2[/latex] | 2 |
| F2 | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\sigma_{2px})^2 (\pi_{2py}, \pi_{2pz})^4 (\pi^*_{2py}, \pi^*_{2pz})^4[/latex] | 1 |
| Ne2 (unstable) | [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\sigma_{2px})^2 (\pi_{2py}, \pi_{2pz})^4 (\pi^*_{2py}, \pi^*_{2pz})^4 (\sigma^*_{2px})^2[/latex] | 0 |
| Table 1. Electron Configuration and Bond Order for Molecular Orbitals in Homonuclear Diatomic Molecules of Period Two Elements | ||
Li2 is analogous to H2, but the AOs involved are the valence 2s orbitals. Each of the two lithium atoms has one valence electron. Hence, we have two valence electrons available for the σ2s bonding MO, and we would predict the Li2 molecule to be stable with a bond order of 1. The molecule is, in fact, present in appreciable concentration in lithium vapor at temperatures near the boiling point of the element. All of the other molecules in Table 1 with a bond order greater than zero are also known.
The O2 molecule has enough electrons to half fill the [latex]\pi^*_{2py}[/latex] and [latex]\pi^*_{2pz}[/latex] MOs. We expect the two electrons that occupy these two degenerate orbitals to be unpaired, and this molecular electronic configuration for O2 is in accord with the fact that the oxygen molecule has two unpaired electrons (see Example 2 below). The unpaired electrons of the oxygen molecule provide a strong piece of experimental evidence for the validity of molecular orbital theory.
Band Theory
When two identical atomic orbitals on different atoms combine, two molecular orbitals result. The bonding molecular orbital is lower in energy than the original atomic orbitals. The antibonding molecular orbital is higher in energy than the original atomic orbitals.
In a solid, similar things happen, but on a much larger scale. Remember that even in a small sample there are a huge number of atoms (typically > 1023 atoms), and therefore a huge number of atomic orbitals that may be combined into molecular orbitals. When N valence atomic orbitals, all of the same energy and each containing one electron, are combined, N/2 (filled) bonding molecular orbitals and N/2 (empty) antibonding molecular orbitals will result. Each bonding MO will show an energy lowering as the atomic orbitals are mostly in-phase, but each of the bonding MOs will be a little different and have slightly different energies. The antibonding MOs will show increase in energy as the atomic orbitals are mostly out-of-phase, but each of the antibonding MOs will also be a little different and have slightly different energies. The allowed energy levels for all the bonding MOs are so close together that they form a band, called the valence band. Likewise, all the antibonding MOs are very close together and form a band, called the conduction band. Figure 13 shows the bands for three important classes of materials: insulators, semiconductors, and conductors.
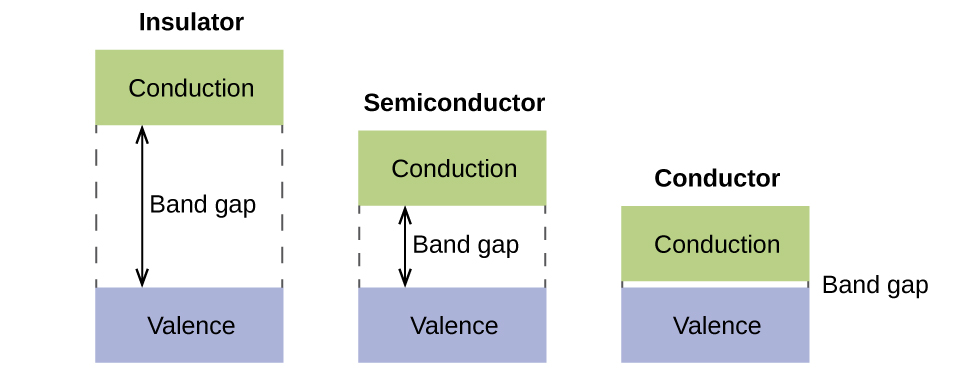
In order to conduct electricity, electrons must move from the filled valence band to the empty conduction band where they can move throughout the solid. The size of the band gap, or the energy difference between the top of the valence band and the bottom of the conduction band, determines how easy it is to move electrons between the bands. Only a small amount of energy is required in a conductor because the band gap is very small. This small energy difference is “easy” to overcome, so they are good conductors of electricity. In an insulator, the band gap is so large that very few electrons move into the conduction band; as a result, insulators are poor conductors of electricity. Semiconductors conduct electricity when “moderate” amounts of energy are provided to move electrons out of the valence band and into the conduction band.
Semiconductors, such as silicon, are used in devices such as computers, smartphones, and solar cells. Solar cells produce electricity when sunlight provides the energy to move electrons out of the valence band. The electricity that is generated may then be used to power a light or tool, or it can be stored for later use by charging a battery. As of December 2014, up to 46% of the energy in sunlight could be converted into electricity using solar cells.
Example 2
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons
Draw the molecular orbital diagram for the oxygen molecule, O2. From this diagram, calculate the bond order for O2. How does this diagram account for the paramagnetism of O2?
Solution
We draw a molecular orbital energy diagram similar to that shown in Figure 11. Each oxygen atom contributes six electrons, so the diagram appears as shown in Figure 14.
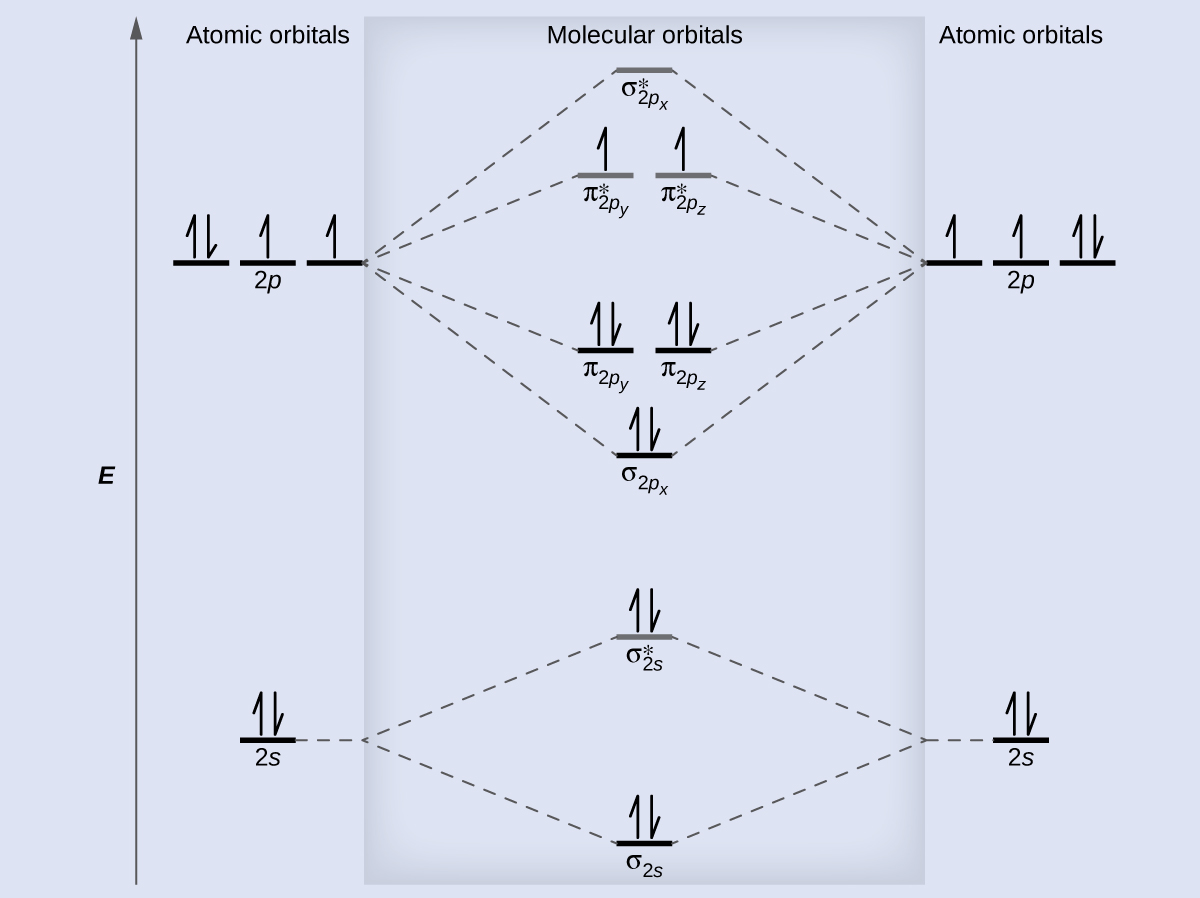
We calculate the bond order as
Oxygen has a double bond, and its paramagnetism is explained by the presence of two unpaired electrons in the π2py* and π2pz* molecular orbitals.
Check Your Learning
The main component of air is N2. From the molecular orbital diagram of N2, predict its bond order and whether it is diamagnetic or paramagnetic.
Answer:
N2 has a bond order of 3 and is diamagnetic.
Example 3
Ion Predictions with MO Diagrams
Give the molecular orbital configuration for the valence electrons in C22−. Will this ion be stable?
Solution
Looking at the appropriate MO diagram, we see that the valence electron configuration for C2 is [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\pi_{2py}, \pi_{2pz})^4[/latex]. Adding two more electrons to generate the C22− anion will give a valence electron configuration of [latex](\sigma_{2s})^2 (\sigma^*_{2s})^2 (\pi_{2py}, \pi_{2pz})^4 (\sigma_{2px})^2[/latex]. Since this has six more bonding electrons than antibonding, the bond order will be 3, and the ion should be stable.
Check Your Learning
How many unpaired electrons would be present on a Be22− ion? Would it be paramagnetic or diamagnetic?
Answer:
two, paramagnetic
Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the diatomic examples presented here. See three-dimensional drawings of the molecular orbitals for C6H6.
Ch4.5 Lewis Symbols and Lewis Structures
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:
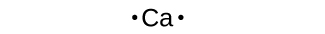
Figure 15 shows the Lewis symbols for the elements of the third period of the periodic table.
![A table is shown that has three columns and nine rows. The header row reads “Atoms,” “Electronic Configuration,” and “Lewis Symbol.” The first column contains the words “sodium,” “magnesium,” “aluminum,” “silicon,” “phosphorus,” “sulfur,” “chlorine,” and “argon.” The second column contains the symbols and numbers “[ N e ] 3 s superscript 2,” “[ N e ] 3 s superscript 2, 3 p superscript 1,” “[ N e ] 3 s superscript 2, 3 p superscript 2,” “[ N e ] 3 s superscript 2, 3 p superscript 3,” “[ N e ] 3 s superscript 2, 3 p superscript 4,” “[ N e ] 3 s superscript 2, 3 p superscript 5,” and “[ N e ] 3 s superscript 2, 3 p superscript 6.” The third column contains Lewis structures for N a with one dot, M g with two dots, A l with three dots, Si with four dots, P with five dots, S with six dots, C l with seven dots, and A r with eight dots.](https://wisc.pb.unizin.org/app/uploads/sites/126/2017/08/CNX_Chem_07_03_3rowLewis.jpg)
Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:

Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:

Figure 16 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds.
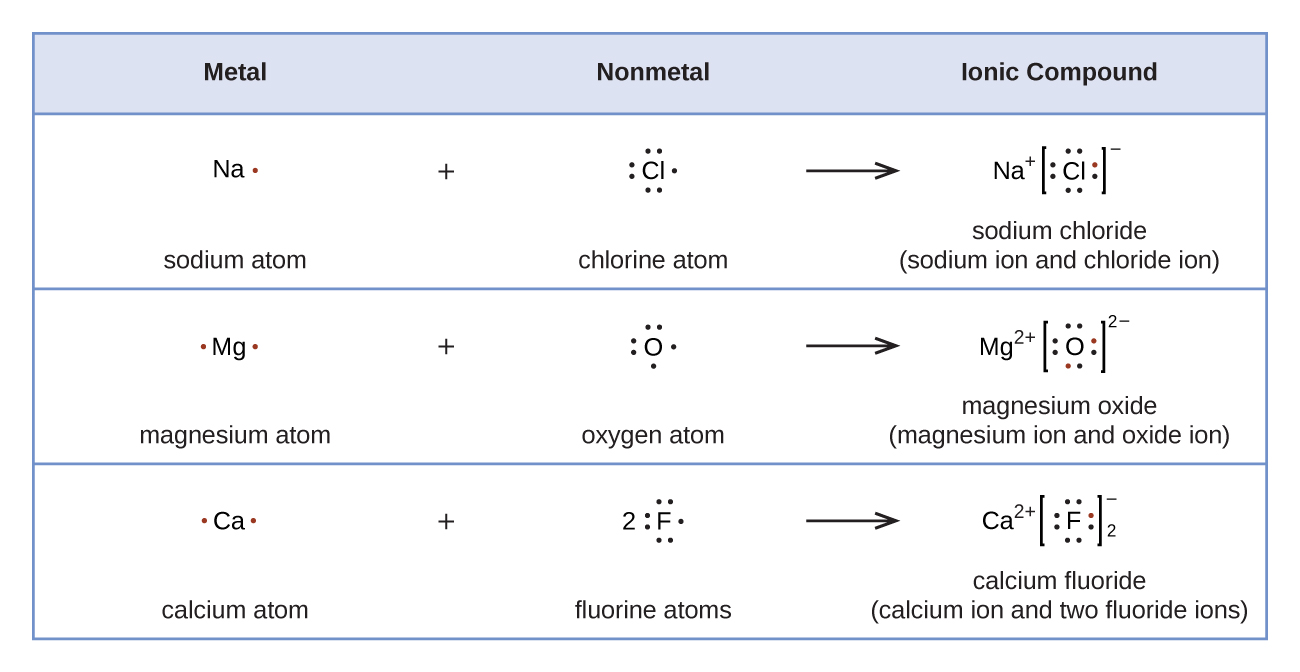
We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and polyatomic ions. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons:
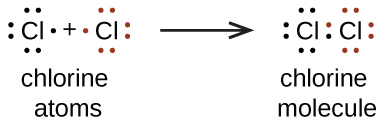
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A line is often used to indicate a shared pair of electrons:

A single shared pair of electrons is called a single bond. Each Cl atom interacts with eight valence electrons: the six in the lone pairs and the two in the single bond.
The Octet Rule
The other halogen molecules (F2, Br2, I2, and At2) form bonds like those in the chlorine molecule: one single bond between atoms and three lone pairs of electrons per atom. This allows each halogen atom to have a noble gas electron configuration. The tendency of main group atoms to form enough bonds to obtain eight valence electrons is known as the octet rule.
The number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight valence electrons); this is especially true of the nonmetals of the second period of the periodic table (C, N, O, and F). For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in CCl4 (carbon tetrachloride) and silicon in SiH4 (silane).
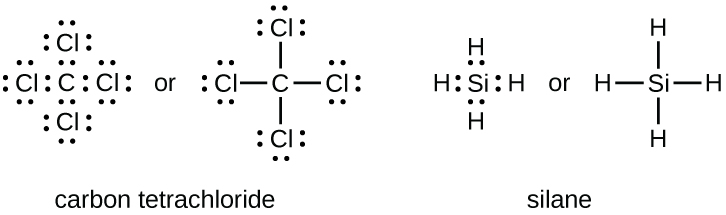
Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule. The transition elements also do not follow the octet rule.
Group 15 elements such as nitrogen have five valence electrons in the atomic Lewis symbol: one lone pair and three unpaired electrons. To obtain an octet, these atoms form three covalent bonds, as in NH3 (ammonia). Oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds:
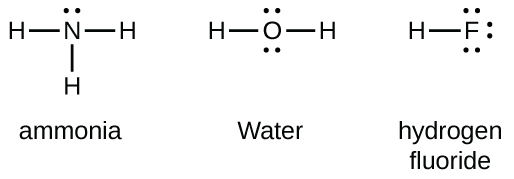
Double and Triple Bonds
A pair of atoms may need to share more than one pair of electrons in order to achieve the requisite octet. A double bond forms when two pairs of electrons are shared between a pair of atoms, as between the carbon and oxygen atoms in CH2O (formaldehyde) and between the two carbon atoms in C2H4 (ethylene):
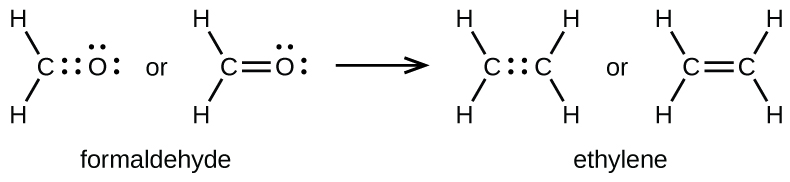
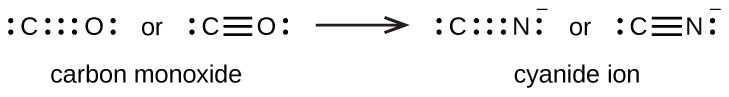
A triple bond forms when three electron pairs are shared by a pair of atoms, as in carbon monoxide (CO) and the cyanide ion (CN–):
Ch4.6 Writing Lewis Structures with the Octet Rule
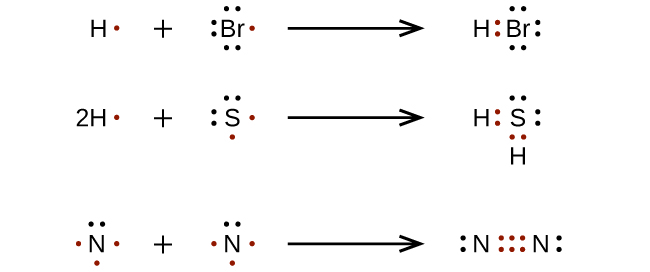
For very simple molecules and molecular ions, we can write the Lewis structures by merely pairing up the unpaired electrons on the constituent atoms. See these examples:
For more complicated molecules and molecular ions, it is helpful to follow a step-by-step procedure such as the one outlined here:
- Determine the total number of valence (outer shell) electrons. For cations, subtract one electron for each positive charge. For anions, add one electron for each negative charge.
- Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom. Connect each atom to the central atom with a single bond (one electron pair).
- Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen), completing an octet around each atom.
- Place all remaining electrons on the central atom.
- Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
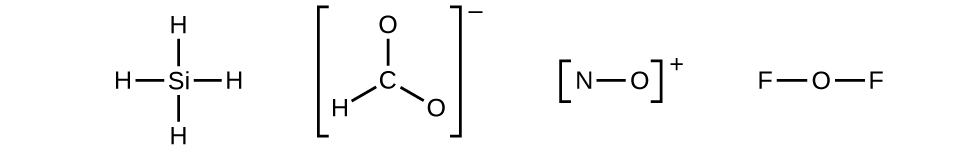
Let us determine the Lewis structures of SiH4, CHO2−, NO+, and OF2 as examples in following this procedure:
- Determine the total number of valence (outer shell) electrons in the molecule or ion.
- For a molecule, such as SiH4, we add the number of valence electrons on each atom in the molecule:
Si: 4 valence electrons/atom x 1 atom = 4
+ H: 1 valence electron/atom x 4 atoms = 4total valence electrons = 8
- For a negative ion, such as CHO2−, we add the number of valence electrons on the atoms to the number of negative charges on the ion (one electron is gained for each single negative charge):
C: 4 valence electrons/atom x 1 atom = 4
+ H: 1 valence electron/atom x 1 atom = 1
+ O: 6 valence electrons/atom x 2 atoms = 12
+ 1 additional electron = 1total valence electrons = 18
- For a positive ion, such as NO+, we add the number of valence electrons on the atoms in the ion and then subtract the number of positive charges on the ion (one electron is lost for each single positive charge) from the total number of valence electrons:
N: 5 valence electrons/atom x 1 atom = 5
+ O: 6 valence electrons/atom x 1 atom = 6
- 1 fewer electron = -1total valence electrons = 10
- Since OF2 is a neutral molecule, we simply add the number of valence electrons:
O: 6 valence electrons/atom x 1 atom = 6
+ F: 7 valence electrons/atom x 2 atoms = 14total valence electrons = 20
- For a molecule, such as SiH4, we add the number of valence electrons on each atom in the molecule:
- Draw a skeleton structure of the molecule or ion, arranging the atoms around a central atom and connecting each atom to the central atom with a single bond (one electron pair). (Note that we denote ions with brackets around the structure, indicating the charge outside the brackets:)
- When several arrangements of atoms are possible, as for CHO2−, we must use experimental evidence to choose the correct one. In general, the less electronegative elements are more likely to be central atoms. In CHO2−, the less electronegative carbon atom occupies the central position with the oxygen and hydrogen atoms surrounding it. Other examples include P in POCl3, S in SO2, and Cl in ClO4−. An exception is that hydrogen is almost never a central atom. As the most electronegative element, fluorine also cannot be a central atom.
- Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen) to complete their valence shells with an octet of electrons.
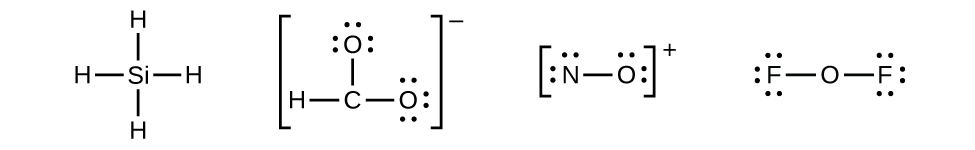
- There are no remaining electrons on SiH4, so it is unchanged.
- Place all remaining electrons on the central atom.
- For SiH4, CHO2−, and NO+, there are no remaining electrons; we already placed all of the electrons determined in Step 1.
- For OF2, we had 16 electrons remaining in Step 3, and we placed 12, leaving 4 to be placed on the central atom:
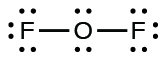
- Rearrange the electrons of the outer atoms to make multiple bonds with the central atom in order to obtain octets wherever possible.
- SiH4: Si already has an octet, so nothing needs to be done.
- CHO2−: We have distributed the valence electrons as lone pairs on the oxygen atoms, but the carbon atom lacks an octet:
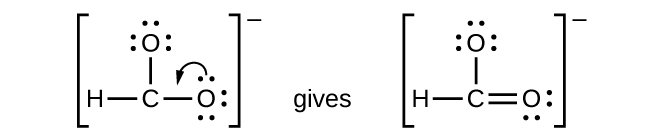
- NO+: For this ion, we added eight valence electrons, but neither atom has an octet. We cannot add any more electrons since we have already used the total that we found in Step 1, so we must move electrons to form a multiple bond:
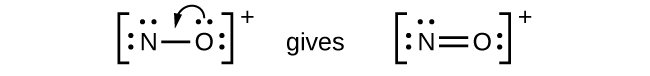
This still does not produce an octet, so we must move another pair, forming a triple bond:
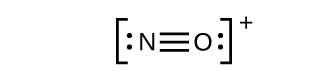
- In OF2, each atom has an octet as drawn, so nothing changes.
Example 4
Writing Lewis Structures
NASA’s Cassini-Huygens mission detected a large cloud of toxic hydrogen cyanide (HCN) on Titan, one of Saturn’s moons. Titan also contains ethane (H3CCH3), acetylene (HCCH), and ammonia (NH3). What are the Lewis structures of these molecules?
Solution
- Calculate the number of valence electrons.
HCN: (1 × 1) + (4 × 1) + (5 × 1) = 10
H3CCH3: (1 × 3) + (2 × 4) + (1 × 3) = 14
HCCH: (1 × 1) + (2 × 4) + (1 × 1) = 10
NH3: (5 × 1) + (3 × 1) = 8 - Draw a skeleton and connect the atoms with single bonds. Remember that H is never a central atom:
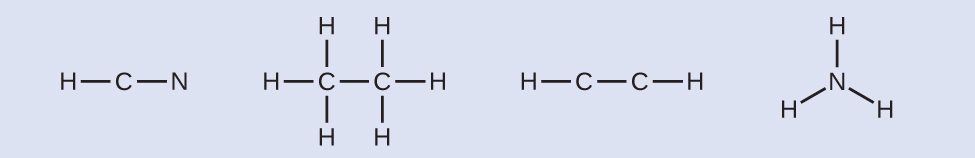
- Where needed, distribute electrons to the terminal atoms:
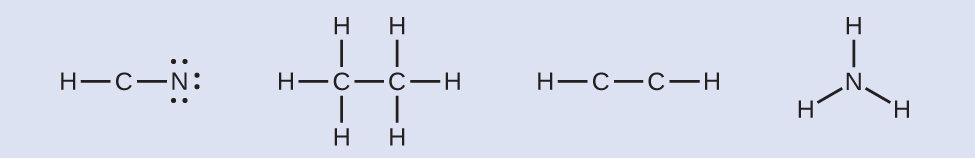 HCN: six electrons placed on N. H3CCH3: no electrons remain. HCCH: no terminal atoms capable of accepting electrons. NH3: no terminal atoms capable of accepting electrons.
HCN: six electrons placed on N. H3CCH3: no electrons remain. HCCH: no terminal atoms capable of accepting electrons. NH3: no terminal atoms capable of accepting electrons. - Where needed, place remaining electrons on the central atom:
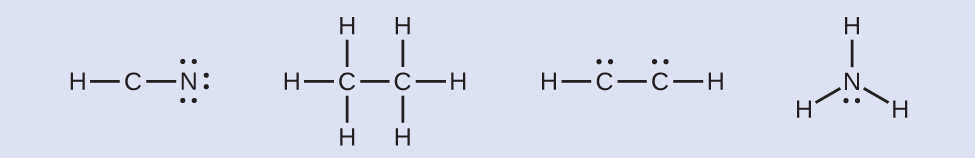 HCN: no electrons remain. H3CCH3: no electrons remain. HCCH: four electrons placed on carbon. NH3: two electrons placed on nitrogen.
HCN: no electrons remain. H3CCH3: no electrons remain. HCCH: four electrons placed on carbon. NH3: two electrons placed on nitrogen. - Where needed, rearrange electrons to form multiple bonds in order to obtain an octet on each atom:
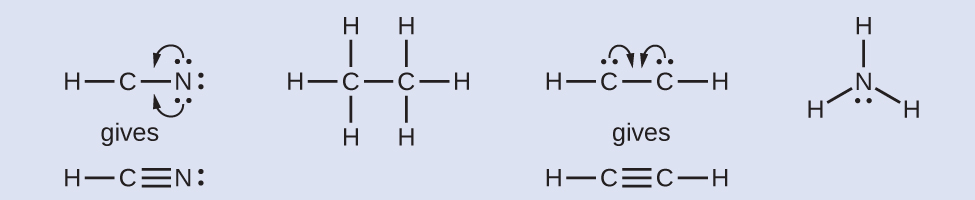 HCN: form two additional C–N bonds. H3CCH3: all atoms have the correct number of electrons. HCCH: form two additional C–C bonds. NH3: all atoms have the correct number of electrons.
HCN: form two additional C–N bonds. H3CCH3: all atoms have the correct number of electrons. HCCH: form two additional C–C bonds. NH3: all atoms have the correct number of electrons.
Check Your Learning
Both carbon monoxide, CO, and carbon dioxide, CO2, are products of the combustion of fossil fuels. Both of these gases also cause problems: CO is toxic and CO2 has been implicated in global climate change. What are the Lewis structures of these two molecules?
Answer:

Ch4.7 Hydrocarbons
The largest database[1] of organic compounds lists about 10 million substances, which include compounds originating from living organisms and those synthesized by chemists. The number of potential organic compounds has been estimated[2] at 1060—an astronomically high number. The existence of so many organic molecules is a consequence of the ability of carbon atoms to form up to four strong bonds to other carbon atoms, resulting in chains and rings of many different sizes, shapes, and complexities.
The simplest organic compounds contain only the elements carbon and hydrogen, and are called hydrocarbons. Even though they are composed of only two types of atoms, there is a wide variety of molecules consist of varying lengths of chains, branched chains, rings, or combinations of these structures. In addition, hydrocarbons may differ in the types of carbon-carbon bonds present in their molecules. Many hydrocarbons are found in plants, animals, and their fossils; other hydrocarbons have been prepared in the laboratory. We use hydrocarbons every day, mainly as fuels, such as natural gas, acetylene, propane, butane, and the principal components of gasoline, diesel fuel, and heating oil. The familiar plastics polyethylene, polypropylene, and polystyrene are also hydrocarbons.
Ch4.8 Alkanes
Alkanes, or saturated hydrocarbons, contain only single covalent bonds between carbon atoms. Each of the carbon atoms in an alkane is bonded to four other atoms, each of which is either carbon or hydrogen. Noncyclic alkanes all have a formula of CnH2n+2.
The Lewis structures and models of methane, ethane, and pentane are illustrated in Figure 17. Carbon chains are usually drawn as straight lines in Lewis structures, and such Lewis structures are not intended to indicate the geometry of molecules. Notice that the carbon atoms in the structural models (the ball-and-stick and space-filling models) of the pentane molecule do not lie in a straight line but has a zigzag shape. We will learn about the 3D geometry of molecules in more detail soon.
The structures of alkanes and other organic molecules may also be represented in a less detailed manner by condensed structural formulas (or simply, condensed formulas). Instead of the usual format for chemical formulas in which each element symbol appears just once, a condensed formula is written to suggest the bonding in the molecule. These formulas have the appearance of a Lewis structure from which most or all of the bond symbols have been removed. Condensed structural formulas for ethane and pentane are shown at the bottom of Figure 17.
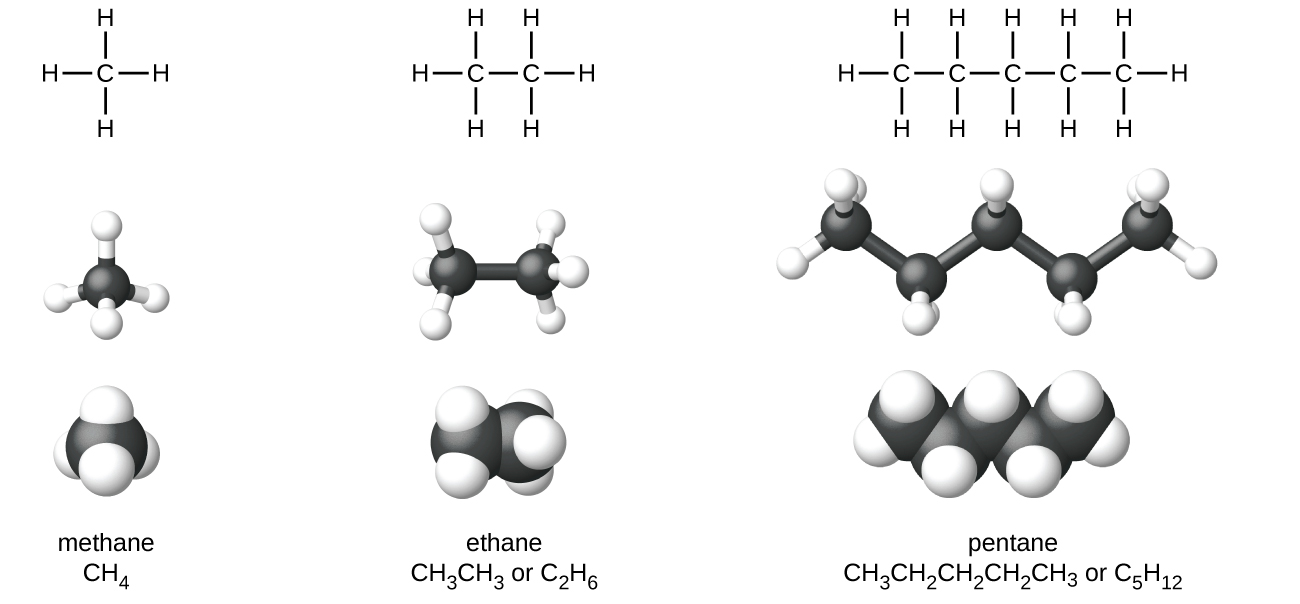
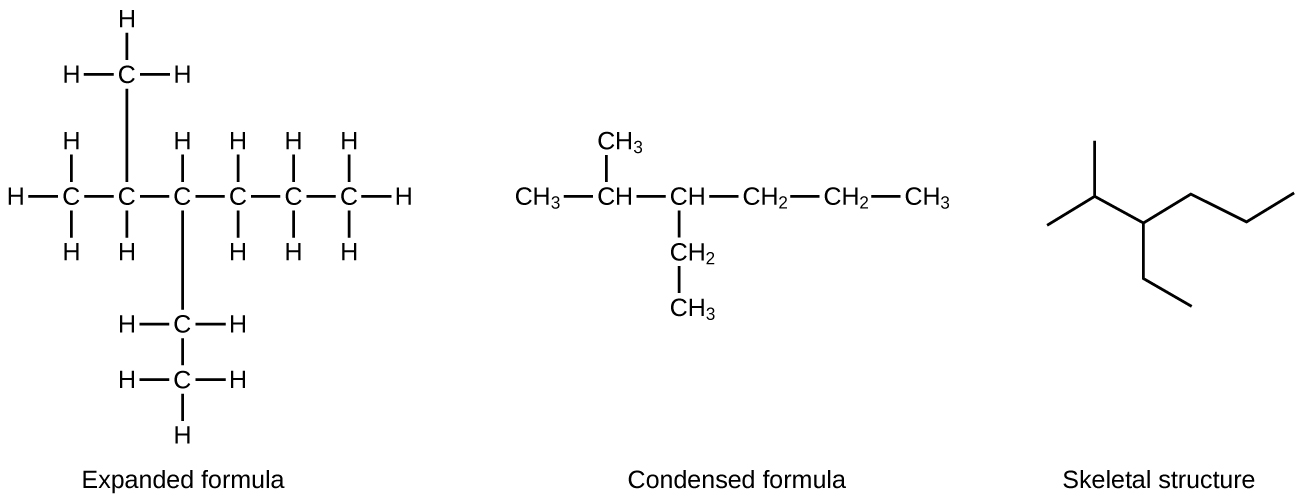
A common method to simplify the drawings of larger molecules is to use a skeletal structure (also called a line-angle structure). In this type of structure, carbon atoms are not symbolized with a C, but represented by each end of a line or bend in a line. Hydrogen atoms are not drawn if they are attached to a carbon. Other atoms besides carbon and hydrogen are represented by their elemental symbols. Figure 18 shows three different ways to draw the same structure.
Example 5
Drawing Skeletal Structures
Draw the skeletal structures for these two molecules:
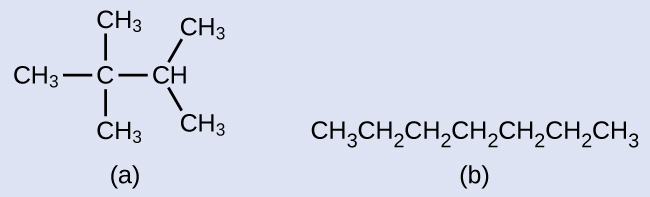
Solution
Each carbon atom is converted into the end of a line or the place where lines intersect. All hydrogen atoms attached to the carbon atoms are left out of the structure (although we still need to recognize they are there):
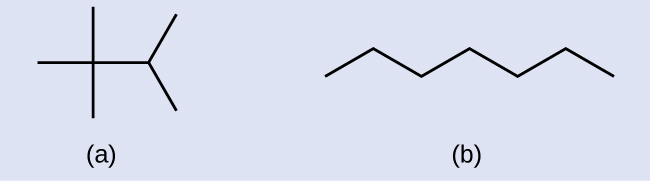
Check Your Learning
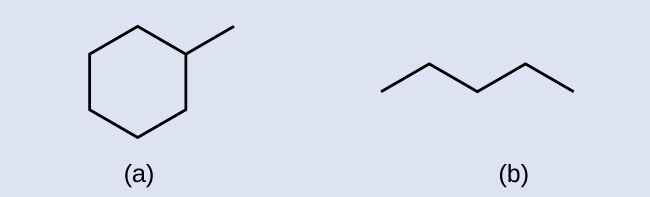
Draw the skeletal structures for these two molecules:
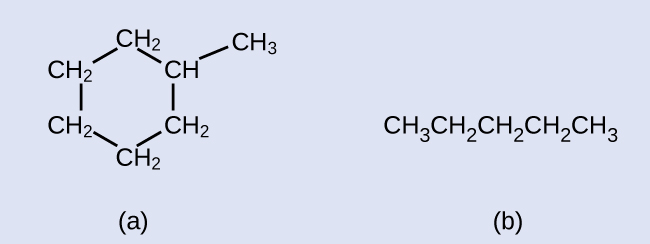
Answer:
Example 6
Interpreting Skeletal Structures
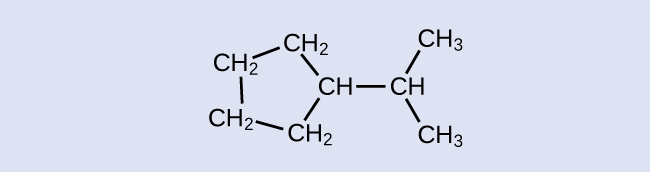
Identify the chemical formula of the molecule represented here:
Solution
There are eight places where lines intersect or end, meaning that there are eight carbon atoms in the molecule. Since we know that carbon atoms tend to make four bonds, each carbon atom will have the number of hydrogen atoms that are required for four bonds. This compound contains 16 hydrogen atoms for a molecular formula of C8H16.
Location of the hydrogen atoms:
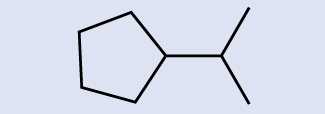
Check Your Learning
Identify the chemical formula of the molecule represented here:
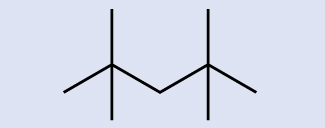
Answer:
C9H20
Constitutional Isomers
Hydrocarbons with the same formula can have different structures. For example, alkanes with the formula C4H10 can have the following Lewis structures, and are called n-butane and 2-methylpropane (or isobutane).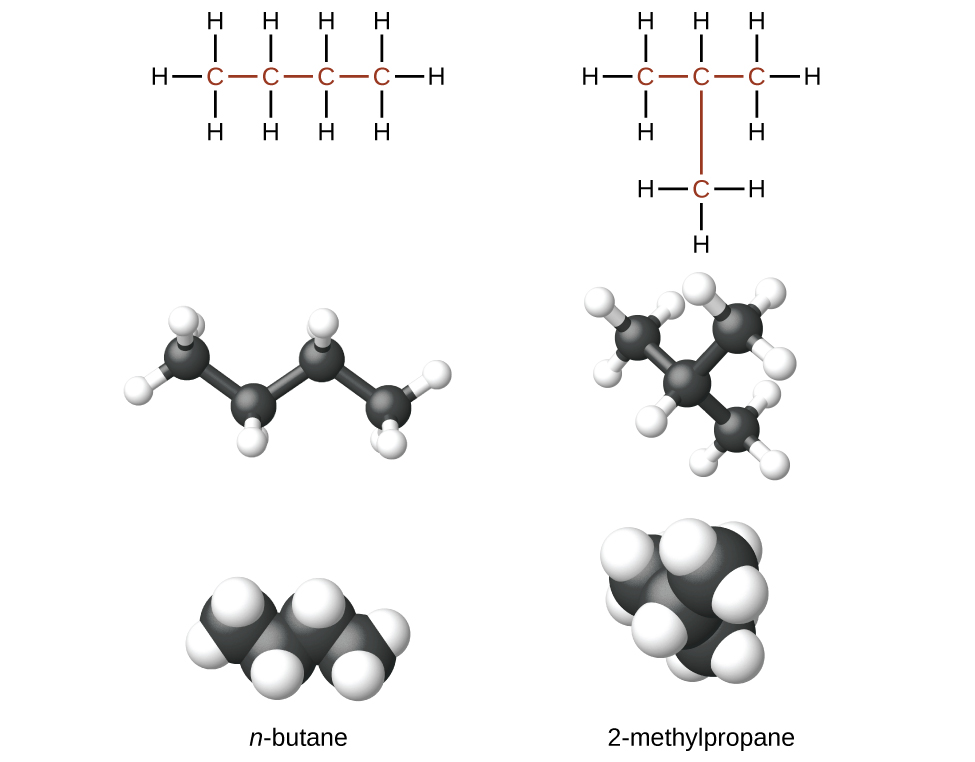
The two compounds are structural isomers (or constitutional isomers). Constitutional isomers have the same molecular formula but different spatial arrangements of the atoms. They differ from one another in one or more physical properties, such as boiling point, solubility, chemical reactivity, etc. The n-butane molecule contains an unbranched chain, meaning that no carbon atom is bonded to more than two other carbon atoms. We use the term normal, or the prefix n, to refer to a chain of carbon atoms without branching. The compound 2–methylpropane has a branched chain (the carbon atom in the center of the Lewis structure is bonded to three other carbon atoms).
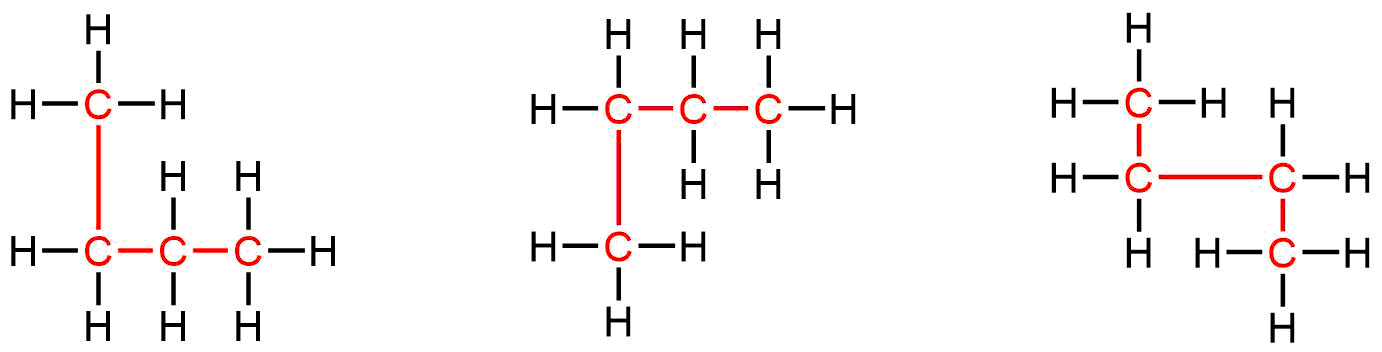
Identifying isomers from Lewis structures is not as easy as it looks. Lewis structures that look different may actually represent the same isomers. For example, the three structures in Figure 19 all represent the same molecule, n-butane, and hence are not different isomers. They are identical because each contains an unbranched chain of four carbon atoms.
Cyclic Alkanes
Cycloalkanes are characterized by a ring of carbon atoms. If a hydrocarbon chain is to be made into a ring, a new C—C bond must be formed between carbon atoms at the end of the chain. This requires that two hydrogen atoms be removed to make room for the new bond. Consequently such a ring involves one more C—C bond and two less C—H bonds than the corresponding normal alkane. Cycloalkanes containing one ring have a general formula of CnH2n, with two less hydrogen atoms than the linear alkane with the same number of carbon atoms. We refer to this reduction in number of hydrogen atoms as degree of unsaturation, which indicates the total number of rings and π bonds present in a molecule. 1 degree of unsaturation corresponding to having two less hydrogen atoms.
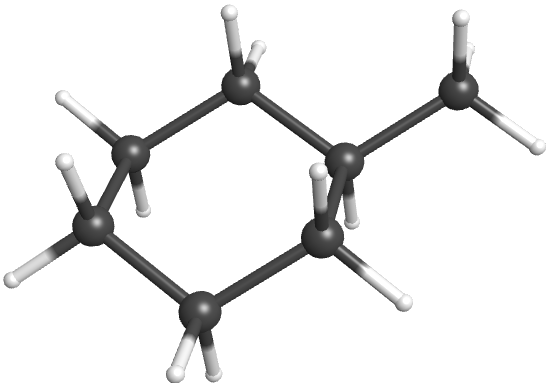
The most common cycloalkane in petroleum is methylcyclohexane:
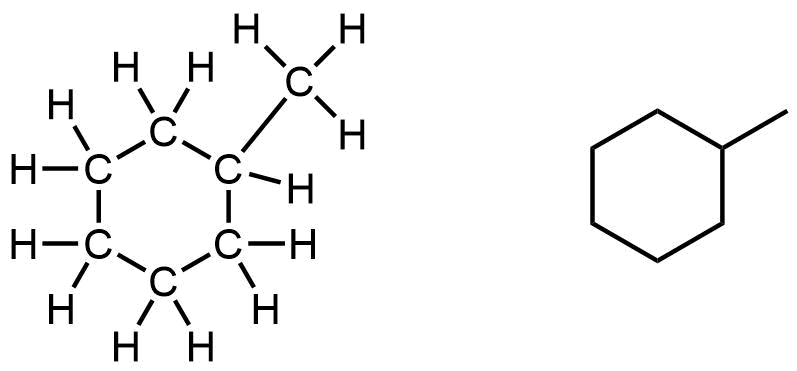
A ball-and-stick model of one of the conformations of methylcyclohexane is shown below. Note that the ring of six carbon atoms is puckered, and does not line in a flat plane.
Ch4.9 Alkenes
Organic compounds that contain one or more double or triple bonds between carbon atoms are unsaturated. You have likely heard of unsaturated fats. These are complex organic molecules with long chains of carbon atoms containing at least one double bond between carbon atoms. Unsaturated hydrocarbon molecules that contain one or more double bonds are called alkenes. Carbon atoms linked by a double bond are bound together by two bonds, one σ bond and one π bond. The general formula for alkenes with one double bond is CnH2n. It has two less hydrogen atoms than the corresponding alkane, and hence 1 degree of unsaturation.
The name of an alkene is derived from the name of the alkane with the same number of carbon atoms. The presence of the double bond is signified by replacing the suffix -ane with the suffix -ene. Ethene, C2H4, commonly called ethylene, is the simplest alkene. The second member of the series is propene (propylene), C3H6. The butene isomers follow in the series. The four carbon atoms in the chain of butene give rise to different constitutional isomers based on the position of the double bond. The location of the double bond is identified by the smaller of the number of the carbon atoms participating in the double bond.
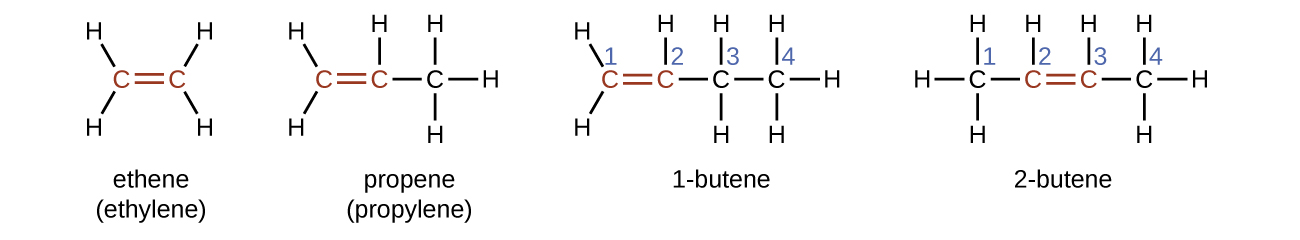
Isomers of Alkenes
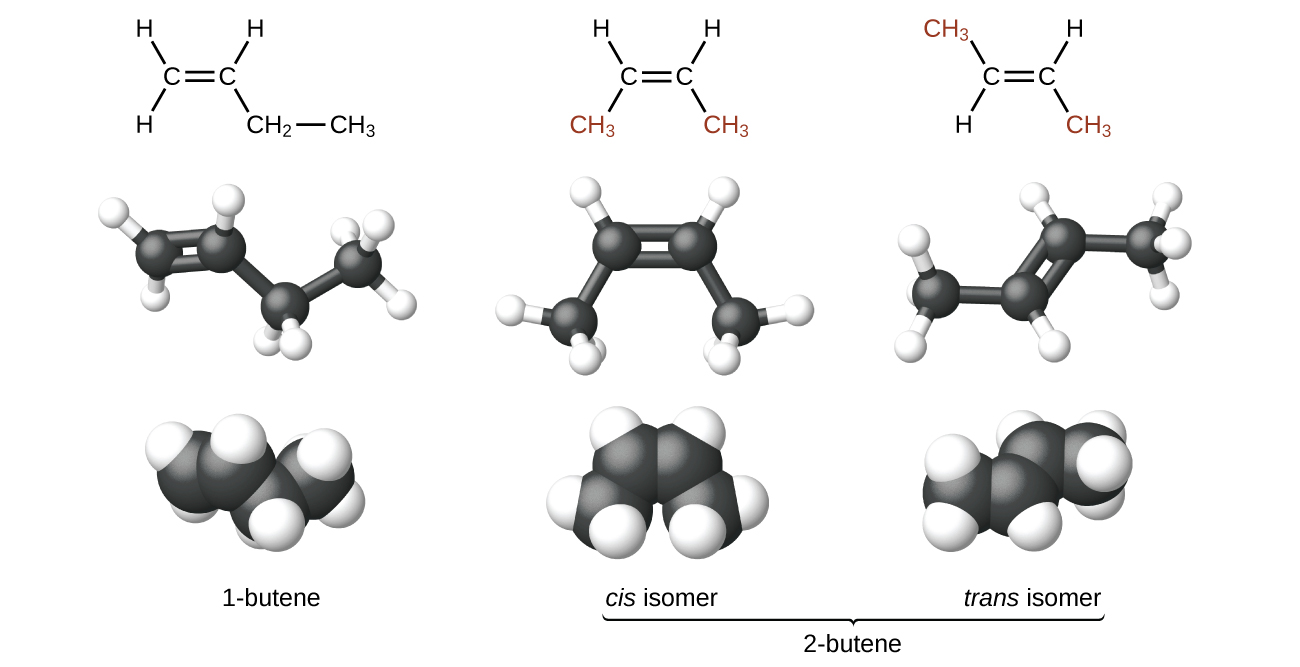
Molecules of 1-butene and 2-butene are constitutional or structural isomers; the arrangement of the atoms in these two molecules differs. For example, the first carbon atom in 1-butene is bonded to two hydrogen atoms; the first carbon atom in 2-butene is bonded to three hydrogen atoms.
2-butene has a second type of isomerism called geometric isomer. In a set of geometric isomers, the same types of atoms are attached to each other in the same order, but the geometries of the two molecules differ. Geometric isomers of alkenes differ in the orientation of the groups on either side of a C=C bond. Carbon atoms are free to rotate around a single bond, but not around a rigid double bond. This makes it possible to have two isomers of 2-butene, one with both methyl groups on the same side of the double bond, called a cis-isomer, and one with the methyl groups on opposite sides, called a trans-isomer. These different geometries produce different physical properties, such as boiling point, that may make separation of geometric isomers possible.
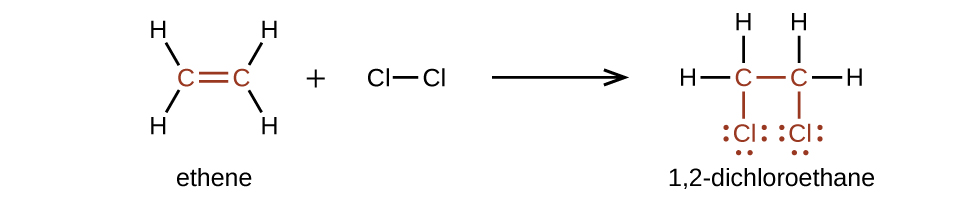
Alkenes are much more reactive than alkanes because the C=C moiety is a reactive functional group. A π bond, being a weaker bond, is disrupted much more easily than a σ bond. Thus, alkenes undergo a characteristic reaction in which the π bond is broken and replaced by two σ bonds. This reaction is called an addition reaction. For example, halogens add to the double bond in an alkene:
Example 7
Alkene Reactivity and Naming
Provide the IUPAC names for the reactant and product of the halogenation reaction shown here:
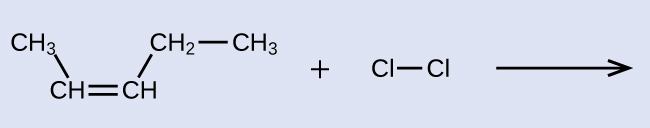
Solution
The reactant is a five-carbon chain that contains a carbon-carbon double bond, so the base name will be pentene. We begin counting at the end of the chain closest to the double bond—in this case, from the left—the double bond spans carbons 2 and 3, so the name becomes 2-pentene. Since there are two carbon-containing groups attached to the two carbon atoms in the double bond—and they are on the same side of the double bond—this molecule is the cis-isomer, making the name of the starting alkene cis-2-pentene. The product of the halogenation reaction will have two chlorine atoms attached to the carbon atoms that were a part of the carbon-carbon double bond:
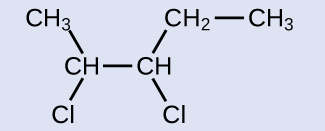
This molecule is now a substituted alkane and will be named as such. The base of the name will be pentane. We will count from the end that numbers the carbon atoms where the chlorine atoms are attached as 2 and 3, making the name of the product 2,3-dichloropentane.
Check Your Learning
What is the product of the reaction shown:
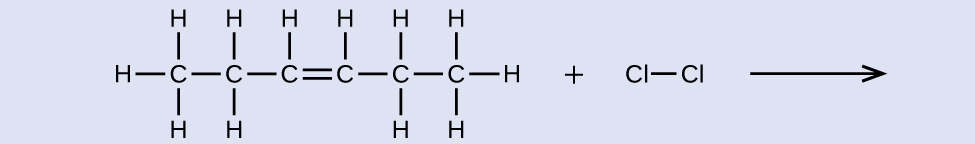
Answer:
3,4-dichlorohexane (can draw the product instead of using its name)
Ch4.10 Alkynes
Hydrocarbon molecules with one or more triple bonds are called alkynes; they make up another series of unsaturated hydrocarbons. Two carbon atoms joined by a triple bond are bound together by one σ bond and two π bonds. The general formula for alkynes with one triple bond is CnH2n-2. It has four less hydrogen atoms than the corresponding alkane, and hence 2 degrees of unsaturation.
The IUPAC nomenclature for alkynes is similar to that for alkenes except that the suffix -yne is used to indicate a triple bond in the chain. The simplest member of the alkyne series is ethyne, C2H2, commonly called acetylene. The Lewis structure for ethyne is:
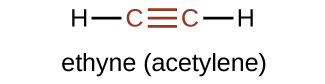
Chemically, the alkynes are similar to the alkenes. Since the C≡C functional group has two π bonds, alkynes typically react even more readily, and react with twice as much reagent in addition reactions. The reaction of acetylene with bromine is a typical example:
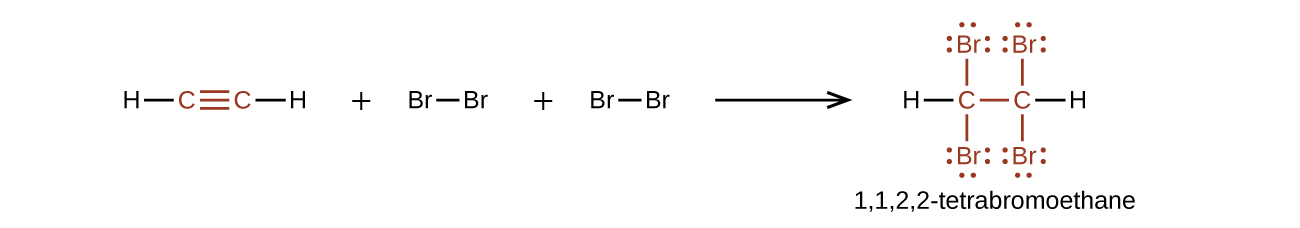
- This is the Beilstein database, now available through the Reaxys site (www.elsevier.com/online-tools/reaxys). ↵
- Peplow, Mark. “Organic Synthesis: The Robo-Chemist,” Nature 512 (2014): 20–2. ↵

