Unit Four
Day 41: Concentration Cells; Batteries
As you work through this section, if you find that you need a bit more background material to help you understand the topics at hand, you can consult “Chemistry: The Molecular Science” (5th ed. Moore and Stanitski) Chapter 17-8 and 17-9, and/or Chapter 17.8-17.11 in the Additional Reading Materials section.
D41.1 Concentration Cells
A concentration cell is a special type of voltaic cell where the electrodes are the same material but the half-cells have different concentrations of soluble species. Because one or both half-cells does not involve standard-state conditions, the half-cell potentials are unequal. Therefore, there is a potential difference between the half-cells. That potential difference can be calculated using the Nernst equation.
For example, consider this cell:
The standard cell potential is 0 V because the anode and cathode involve the same reaction; however, the process is spontaneous because if equal volumes of the two half-cell solutions were mixed, the concentration of Zn2+ would change to the average of the initial concentrations, namely, to 0.30 M. The cell can do work because the concentrations of Zn2+ change.
The Nernst equation verifies that the process is spontaneous at the given conditions, and Ecell > 0 V:
In a concentration cell, the standard cell potential is always zero. To get a positive cell potential (spontaneous process) the reaction quotient Q must be < 1. As the reaction proceeds, Q approaches 1 and Ecell approaches 0 V.
D41.2 Batteries
A battery is an electrochemical cell or series of cells that produces an electric current. In principle, any voltaic cell can be used as a battery. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Real batteries strike a balance between ideal characteristics and practical limitations.
For example, the mass of a car battery is about 18 kg or ~1% of the mass of an average car. This type of battery would supply nearly unlimited energy if used in a smartphone, but would be completely impractical because of its mass. Thus, no single battery is “best” and batteries are selected for a particular application, keeping things like its mass, cost, reliability, and current capacity in mind.
There are two basic types of batteries: primary and secondary. A few batteries of each type are described next.
D41.3 Primary Batteries
Primary batteries are single-use batteries that cannot be recharged. A common primary battery is the dry cell (Figure 1), which is a zinc-carbon battery. The zinc serves as both a container and the negative electrode. The positive electrode is a rod made of carbon that is surrounded by a paste of manganese(IV) oxide, zinc chloride, ammonium chloride, carbon powder, and a small quantity of water.
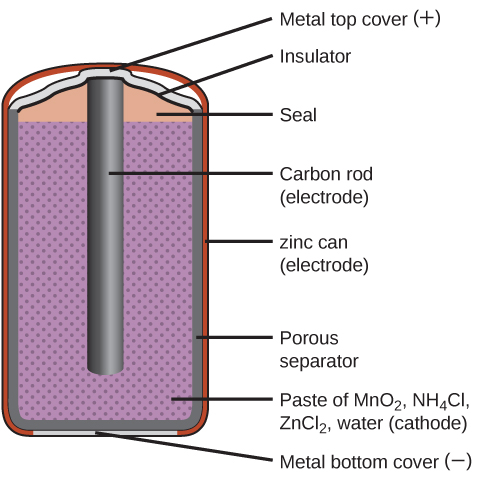
The reaction at the anode can be represented as the ordinary oxidation of zinc:
The reaction at the cathode is more complicated, in part because more than one reduction reaction occurs. The series of reactions that occurs at the cathode is approximately:
The overall reaction for the zinc–carbon battery can be represented as:
The cell potential is about 1.5 V initially, and decreases as the battery is used. As the zinc container oxidizes, its contents eventually leak out, so this type of battery should not be left in any electrical device for extended periods.
The voltage delivered by a battery is the same regardless of the size of a battery. For this reason, D, C, A, AA, and AAA batteries all have the same voltage. However, larger batteries can deliver more moles of electrons and will therefore last longer if powering the same device.
Alkaline batteries (Figure 2) were developed in the 1950s partly to address some of the performance issues with zinc–carbon dry cells, and are manufactured to be their exact replacements. As their name suggests, these types of batteries use alkaline electrolytes, often potassium hydroxide. The reactions are:
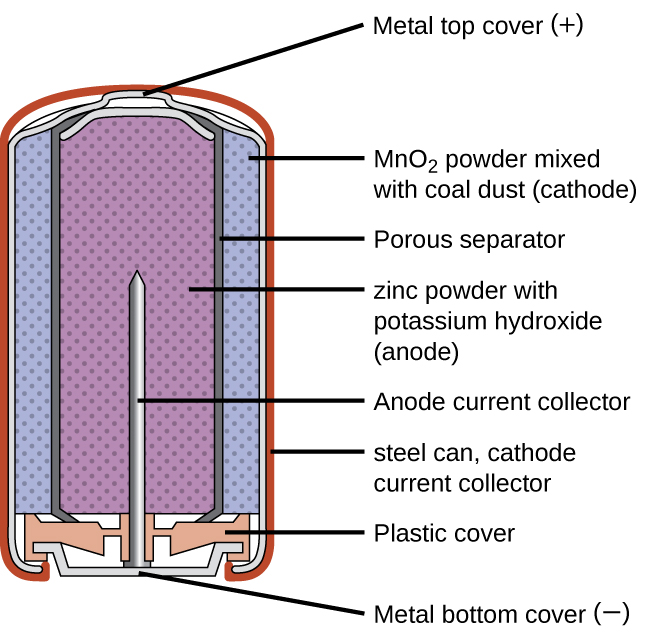
An alkaline battery can deliver about three to five times the energy of a zinc-carbon dry cell of similar size. Alkaline batteries sometimes leak potassium hydroxide, so these should also be removed from devices for long-term storage. While some alkaline batteries are rechargeable, most are not. Attempts to recharge an alkaline battery that is not rechargeable often leads to rupture of the battery and leakage of the potassium hydroxide electrolyte.
D41.4 Lead-Acid Batteries
Secondary batteries are rechargeable; that is, the reaction that powers the battery can be reversed so that the original reactants can be regenerated. Secondary batteries are found in smartphones, electronic tablets, automobiles, and many other devices.
The lead-acid battery (Figure 3) is the type of secondary battery used to start automobiles. It is inexpensive and capable of producing the high current required by the starter motors. The reactions for a lead acid battery are:
Each cell produces 2 V, so six cells can be connected in series to produce a 12-V car battery.
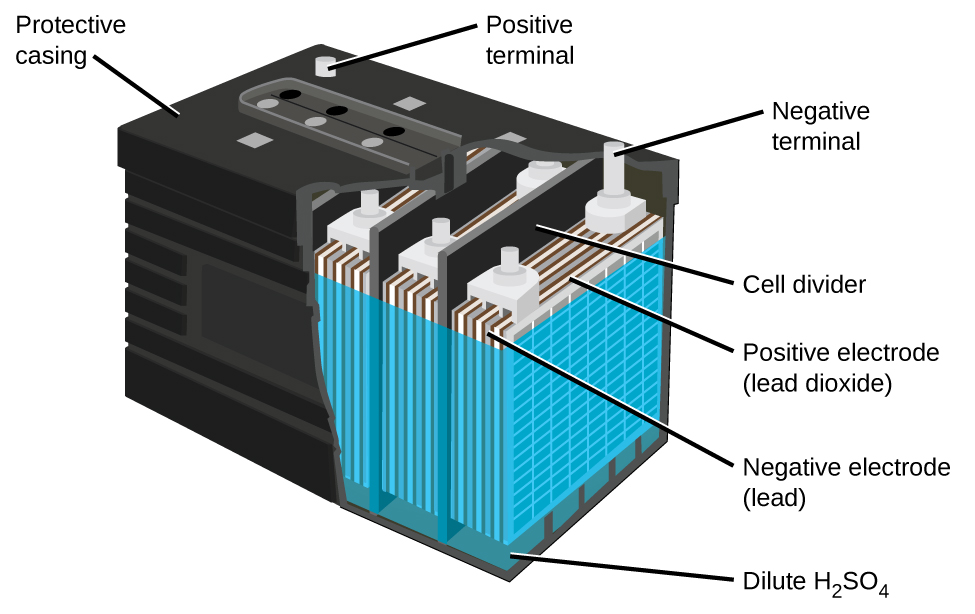
Lead-acid batteries are heavy because of lead’s high density, they contain highly corrosive concentrated sulfuric acid, and must be disposed of properly to avoid lead-poisoning hazards, but they can produce a lot of current in a short time so for certain applications they are the best choice.
Exercise 1: Advantages of Lead-acid Batteries
D41.5 Lithium Ion Batteries
Lithium ion batteries (Figure 1) are among the most popular rechargeable batteries and are used in many portable electronic devices. The reactions are:
(x is no more than about 0.5 moles.) The battery voltage is about 3.7 V.
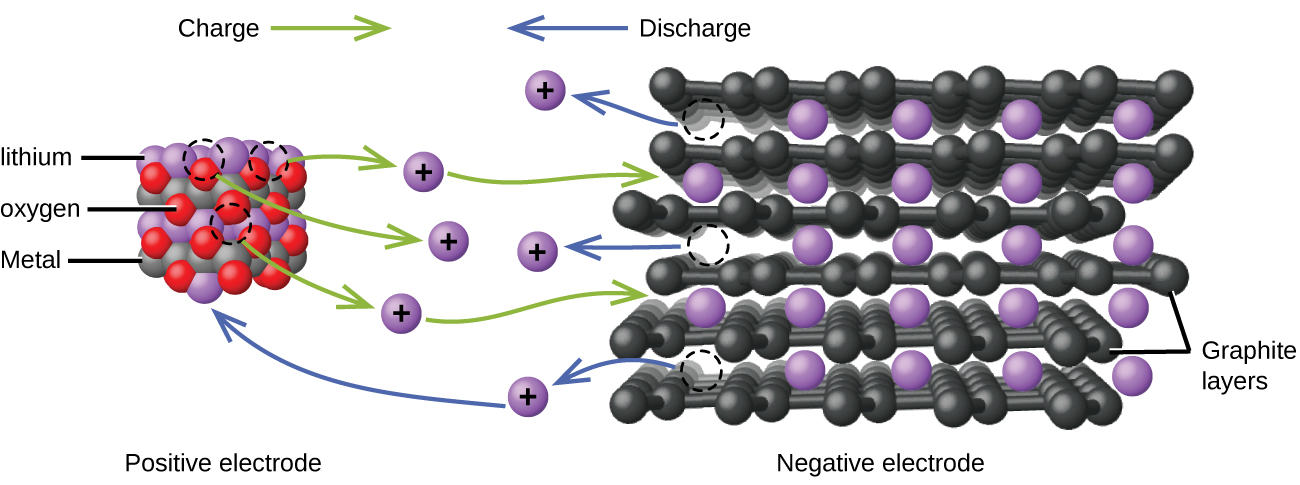
Lithium batteries are popular because they can provide a large amount of current, are lighter than comparable batteries of other types, produce a nearly constant voltage as they discharge, and only slowly lose their charge when stored.
Exercise 2: Lithium-Ion Batteries
Podia Question
Concentration cells are described in Section D41.1 above. A concentration cell is made at 25 °C by placing a Zn electrode at the bottom of a beaker, running an insulated wire from the electrode to the top of the beaker, and covering the electrode with 500. mL 0.50-M ZnSO4(aq). A piece of filter paper the same diameter as the beaker is floated on the 0.50-M ZnSO4 solution, 500. mL of a less concentrated ZnSO4 solution is poured carefully onto the filter paper (so that the two solutions do not mix), and a Zn electrode is suspended in the upper solution. The cell generates 0.10 V. Calculate the concentration of the second solution.
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

