Unit One
Day 8: More About Hydrocarbons
As you work through this section, if you find that you need a bit more background material to help you understand the topics at hand, you can consult “Chemistry: The Molecular Science” (5th ed. Moore and Stanitski) Chapter 2-9 and Chapter 6-5, and/or Chapter 4.8-4.10 in the Additional Reading Materials section.
D8.1 Line Structures
Activity 1: Reflection
Hydrocarbon molecules can have lots of carbon atoms. For example, molecules of hydrocarbons in motor oil for automobiles contain between 16 and 20 carbon atoms. Drawing Lewis structures for such large molecules takes time, so a simpler drawing, called a line structure, is often used. In a line structure (also called a skeletal structure) for a hydrocarbon, only the C–C bonds are shown. Thus, carbon atoms are represented by end of a line or the angle between two lines; hydrogen atoms and C–H bonds are omitted. (The angles between C–C bonds are similar to the zig-zags seen in models of hydrocarbons.) In line structures, atoms other than carbon and hydrogen are represented by their elemental symbols. Figure 1 shows an example line structure; count the number of C and H atoms in the line structure to verify it represents the same molecule as the Lewis structure.
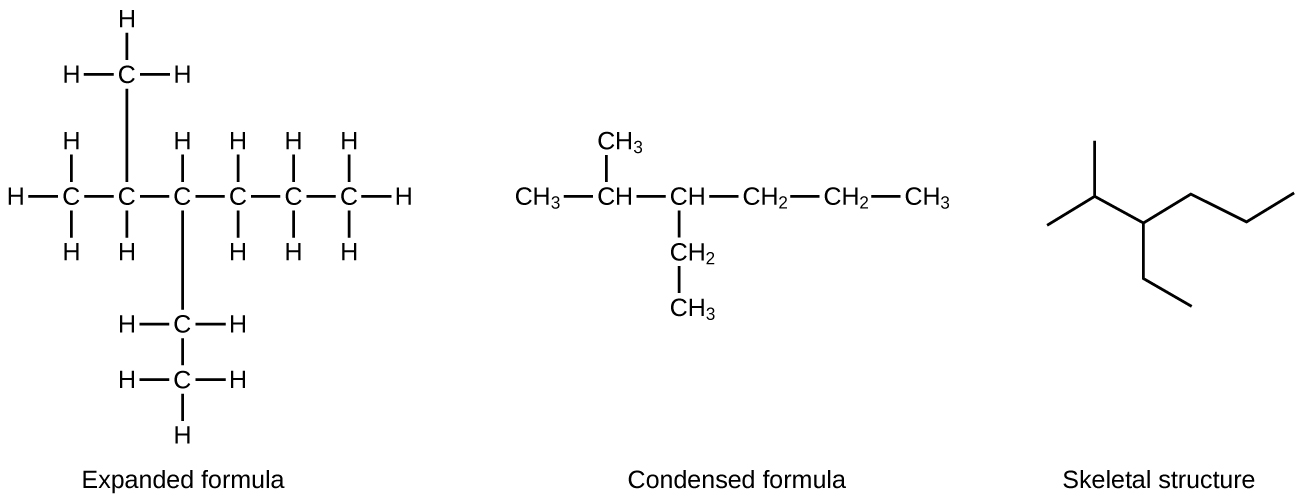
Exercise 1: Interpreting Line Structures
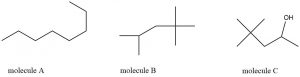
Determine the molecular formula corresponding to each line structure.
D8.2 Cycloalkanes
A cycloalkane has at least one ring of carbon atoms. Comparing a chain of C atoms with a ring shows that an additional C—C bond must be formed. This requires that two hydrogen atoms be removed to make room for the new bond. Consequently such a ring involves one more C—C bond and two less C—H bonds than the corresponding normal alkane, and the general formula for a cycloalkane containing one ring is CnH2n. We refer to this reduction in number of hydrogen atoms as degree of unsaturation, One degree of unsaturation corresponds to having two less hydrogen atoms.
The most common cycloalkane in petroleum is methylcyclohexane:
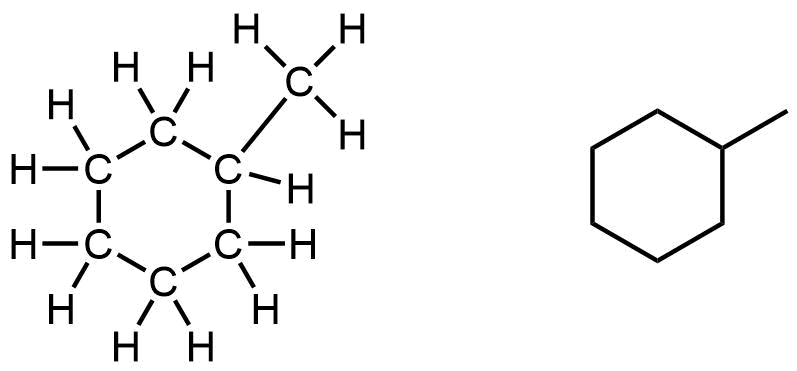
Note that in the 3D ball-and-stick model of one of the conformations of methylcyclohexane, the ring of six carbon atoms is puckered and does not lie flat in a plane.
D8.3 Alkenes
Unsaturated hydrocarbons that contain one or more double bonds are called alkenes. Carbon atoms linked by a double bond are bound together by two bonds: one σ bond and one π bond. The general formula for alkenes with one double bond is CnH2n. The formula has two less hydrogen atoms than the corresponding alkane, and hence 1 degree of unsaturation. It is possible to have a ring of carbon atoms that contains a double bond. Cyclic alkenes have one degree of unsaturation from each cyclic structure and one from each C=C double bond.
Exercise 2: Degree of Unsaturation
The presence of the double bond is signified by the suffix -ene in the name. Ethene, C2H4, commonly called ethylene, is the simplest alkene. The four carbon atoms in the chain of butene give rise to two different constitutional isomers, 1-butene and 2-butene, where the number indicates the position of the double bond.
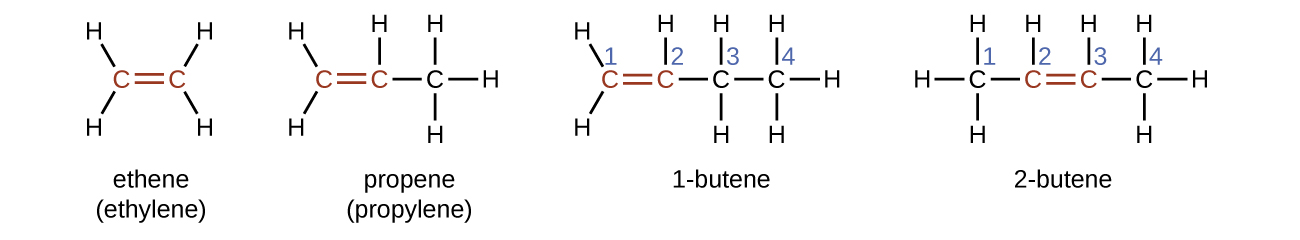
The presence of the π bond make alkenes much more reactive than alkanes because a π bond is usually weaker and more easily disrupted than a σ bond. The double bond is therefore a functional group, a specific structure that has the same chemical behavior in every molecule where it occurs. For example, all alkenes can undergo a characteristic reaction in which the π bond is broken and replaced by two additional σ bonds. This reaction is called an addition reaction. Hydrogen and halogens can add to the double bond in an alkene. Here is an addition reaction involving chlorine:
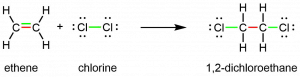
Activity 2: Analyzing an Addition Reaction
- In the equation just above, two bonds are color-coded green in reactants and products. In your notebook, explain the significance of the color coding for chemical bonding and reactivity.
- Think about the reaction of chlorine with ethane, CH3CH3. Can this be an addition reaction? Explain why or why not. What bonds need to be broken and formed if chlorine reacts with ethane and how does the reaction differ from the reaction of chlorine with ethene shown above? Do you expect the reaction of chlorine with ethane to be faster or slower than the reaction with ethene? Why?
D8.4 Geometric Isomers
Because of the additional presence of a π bond, the two carbon atoms in a C=C double bond cannot freely rotate with respect to each other. Consider the π bond in ethene, as shown in Figure 2. If one carbon rotates by 90° around the internuclear axis, the 2p AO that forms the bonding π MO can no longer overlap with the 2p AO from the other carbon, and the π bond is broken; the σ bond is still present. Breaking the π bond requires an increase in energy (makes the molecule less stable).
Figure 2. The pi MO in ethene is shown. Move the slider to see the two p orbitals that overlap to form the pi bond and how their overlap disappears when the front of the molecule is rotated 90°. Hover over each diagram for a description.
Because rotation requires breaking the π bond, at room temperature there is not free rotation around a C=C double bond—unlike the rotation around C-C single bonds. This gives rise to geometric isomers, molecules that have the same molecular formula and the same atomic connectivity but differ in the orientation of the groups connected to a C=C bond. For example, there are three isomers of butene: 1-butene and 2-butene are constitutional (structural) isomers; for 2-butene, there are two geometric isomers, cis-2-butene and trans-2-butene. As shown in Figure 3, the isomer with both methyl groups on the same side of the double bond is called a cis isomer (both methyl groups are on the bottom side in Figure 3). The one with the methyl groups on opposite sides is called a trans isomer. Geometric isomers have different physical properties, such as boiling point, that make separating them possible.
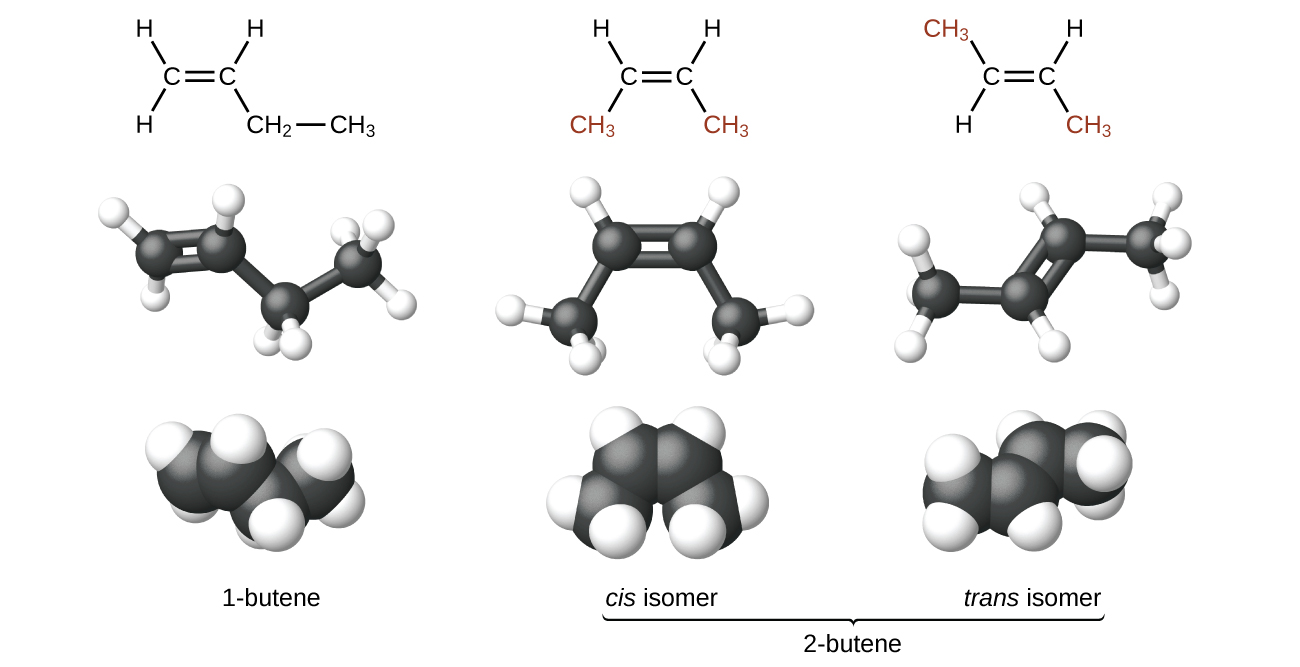
Exercise 3: Isomers
Exercise 4: Addition Reaction Product
Provide the IUPAC name for the alkene reactant and for the saturated product of the addition reaction shown here:
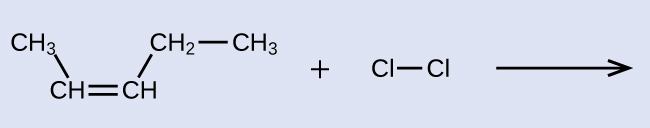
D8.5 Alkynes
An alkyne is a hydrocarbon molecule with one or more triple bonds. Two carbon atoms joined by a triple bond are bound together by one σ bond and two π bonds. The general formula any alkyne with one triple bond is CnH2n-2. The alkyne has four less hydrogen atoms than the corresponding alkane, and hence 2 degrees of unsaturation.
The suffix -yne is used to indicate the presence of a triple bond. The simplest alkyne is ethyne, C2H2, commonly called acetylene. The Lewis structure for ethyne is:
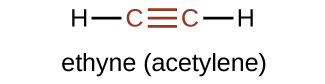
Chemically, alkynes have similar reactivity as alkenes. Since the C≡C functional group has two π bonds, alkynes can react with twice as much reagent in addition reactions. For example, acetylene can react with bromine in the following reaction:
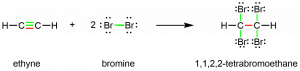
Exercise 5: Formulas, Multiple Bonds, and Rings
Activity 3: Wrap-up
Podia Question
Write a Lewis structure or a line structure for the product of each reaction shown below. (In each case assume there is an excess of bromine.) Compare the products of the second through fifth reactions with the product of the first reaction. Classify each other product as an isomer of, the same as, or neither the same nor an isomer of the first structure.
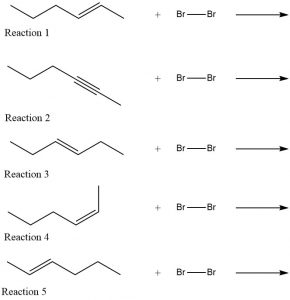
Two days before the next whole-class session, this Podia question will become live on Podia, where you can submit your answer.

